Abstract
Introduction
SCALE-UP II aims to investigate the effectiveness of population health management interventions using text messaging (TM), chatbots and patient navigation (PN) in increasing the uptake of at-home COVID-19 testing among patients in historically marginalised communities, specifically, those receiving care at community health centres (CHCs).
Methods and analysis
The trial is a multisite, randomised pragmatic clinical trial. Eligible patients are >18 years old with a primary care visit in the last 3 years at one of the participating CHCs. Demographic data will be obtained from CHC electronic health records. Patients will be randomised to one of two factorial designs based on smartphone ownership. Patients who self-report replying to a text message that they have a smartphone will be randomised in a 2×2×2 factorial fashion to receive (1) chatbot or TM; (2) PN (yes or no); and (3) repeated offers to interact with the interventions every 10 or 30 days. Participants who do not self-report as having a smartphone will be randomised in a 2×2 factorial fashion to receive (1) TM with or without PN; and (2) repeated offers every 10 or 30 days. The interventions will be sent in English or Spanish, with an option to request at-home COVID-19 test kits. The primary outcome is the proportion of participants using at-home COVID-19 tests during a 90-day follow-up. The study will evaluate the main effects and interactions among interventions, implementation outcomes and predictors and moderators of study outcomes. Statistical analyses will include logistic regression, stratified subgroup analyses and adjustment for stratification factors.
Ethics and dissemination
The protocol was approved by the University of Utah Institutional Review Board. On completion, study data will be made available in compliance with National Institutes of Health data sharing policies. Results will be disseminated through study partners and peer-reviewed publications.
Trial registration number
ClinicalTrials.gov: NCT05533918 and NCT05533359.
Keywords: COVID-19, Health Equity, Health informatics, Information technology, Public health
Strengths and limitations of this study.
Uses scalable population health management interventions to increase the reach and uptake of at-home COVID-19 testing.
Dissemination strategy modalities (ie, voice and text cellphones) are nearly ubiquitous among adults in the USA, including among historically marginalised populations.
The study relies on self-reported data for its primary outcome (use of at-home testing).
The patient population will be drawn from community health centres that opted to participate in this study, all of which are located in a single state in the USA, which limits generalisability.
Introduction
Racial/ethnic minorities, low socioeconomic status (SES) and rural populations suffer profound health inequities across a wide variety of conditions, including a higher rate of hospitalisation and mortality due to COVID-19.1–4 Similar inequities have been found across the USA for vaccination rates between urban and rural,5 high and low SES6 and white and non-white populations.7 8 Low vaccination rates and withdrawal of protection measures leave historically marginalised populations at high risk for local outbreaks and more contagious variants.
Although public health agencies worldwide have declared the end of the pandemic, timely testing is still important to help reduce exposure and offer timely treatment to individuals at a higher risk for severe disease. However, historically marginalised communities lacked easy and convenient access to testing throughout the COVID-19 pandemic, especially after the closure of mass test sites nationwide.9 10 Although several Food and Drug Administration-approved at-home tests are available, providing a convenient, quick and low-cost alternative for patients to test at home,11 12 substantial disparities exist in the use of at-home COVID-19 testing. While the use of at-home COVID-19 testing has more than tripled between the Delta and Omicron outbreaks, use of at-home testing was more than twice as high among individuals identifying as white, having high SES and having a postgraduate degree.13 Thus, scalable approaches are needed to promote the uptake of at-home COVID-19 testing among individuals from historically marginalised communities.
Despite evidence of a digital divide between high-resource healthcare systems and low-resource community health centres (CHCs),14 15 historically marginalised populations have almost universal access to technology such as cellphones, which provide opportunities for large-scale population health management (PHM) interventions. Even in households with annual incomes less than US$30 000, 97% own a cellphone and 76% own a smartphone.16 The SCALE-UP II trial will investigate three PHM interventions (text messaging (TM), automated chatbot and patient navigation (PN)) to increase the reach and uptake of at-home COVID-19 testing among patients who receive care at CHCs.
Methods and analysis
This protocol was developed using the Standard Protocol Items: Recommendations for Interventional Trials.17 The protocol was approved by the Institutional Review Board (IRB) at the University of Utah on 10 June 2022 (IRB_00150669). The trial was registered with Clinicaltrials.gov (NCT05533359 for patients self-reporting that they have a smartphone and NCT05533918 for all other patients) on 9 September 2022. Enrolment was planned to begin in December 2022 and data collection was planned to end in November 2023.
Patient and public involvement
SCALE-UP II is conducted in partnership with the Association for Utah Community Health (AUCH), CHCs across Utah, the Utah Department of Health and Human Services (UDHHS) and the University of Utah. Our research-practice partnership uses a multipronged community engagement approach to (1) identify research questions, (2) develop, adapt and implement interventions and (3) inform dissemination plans.18 19 The community engagement approach includes a weekly project meeting with AUCH, UDHHS and the research team and quarterly Patient Advisory Committee (PAC; consisting of CHC patient representatives) and Study Advisory Committee (consisting of patients, CHC staff, UDHHS and AUCH representatives) meetings. The research objectives of SCALE-UP II were identified in partnership with AUCH and UDHHS; both AUCH and UDHHS were interested in addressing the impact of COVID-19 among historically marginalised communities in Utah. Input from the PAC informed the design of the text messaging and chatbot interventions. Furthermore, TM and chatbot scripts were developed following information gathered from community members in Utah.
Study design
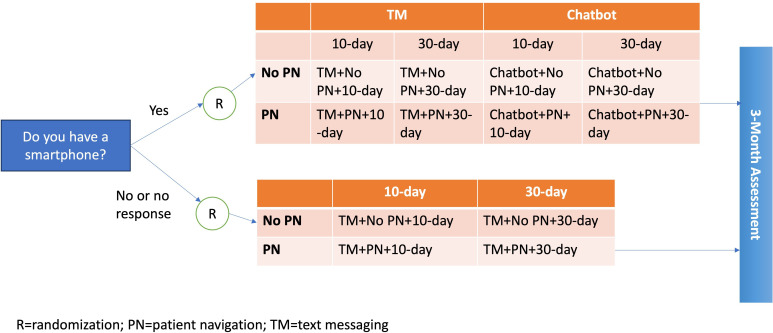
SCALE-UP II is an individually randomised, multisite, pragmatic clinical trial. The experimental design varies according to each patient’s response to a text message asking if they have a smartphone. Participants who self-report that they have a smartphone will be randomised in a 2×2×2 factorial fashion to receive (1) chatbot or TM; (2) PN (yes or no); and (3) repeated offers to interact with the interventions every 10 or 30 days. Participants who do not respond to the introductory text message or who self-report as not having a smartphone will be randomised in a 2×2 factorial fashion to receive (1) TM with or without PN; and (2) repeated offers every 10 or 30 days.
Rationale for study design
The interventions in SCALE-UP II interventions leverage (1) wide adoption of electronic health record (EHR) systems, even in low resource CHCs20 21; (2) wide adoption of cellphones with at least voice and text capabilities16; and (3) the low cost, efficiency and simplicity of at-home COVID-19 tests.11 12 Therefore, SCALE-UP II is designed to maximise reach with low-cost interventions to increase the uptake of COVID-19 testing in historically marginalised communities.
SCALE-UP II will enrol patients from Utah CHCs. These settings provide primary care to diverse, low SES populations and provide an ideal setting to address COVID-19 because there is an established relationship and coordination of care, and ~80% of individuals see a primary care provider at least annually.22 Three Utah CHC systems and their 12 primary care clinics will participate in SCALE-UP II. Demographics of SCALE-UP II CHC patients include: 51% Latino, 62% <100% federal poverty level, 69% uninsured and receiving care in clinics where 17% are in rural/frontier areas.23
Since the chatbot requires a smartphone with a connection to the internet, and about 25% of individuals with low SES and from rural areas do not have a smartphone,16 SCALE-UP II will enrol patients in one of two factorial designs based on their smartphone ownership in order to maximise reach to the 96% of individuals who own at least a voice and text cellphone.
Patients may be reluctant to test due to hesitancy and numerous other barriers.24 25 However, practical advice from patient navigators such as community health workers (CHWs) can help overcome hesitancy and engagement barriers such as logistics, transportation and expense; of critical importance, providers welcome the use of these approaches with their patients.26–28 Thus, in addition to the TM and chatbot interventions, SCALE-UP II will examine the added effect of offering access to patient navigation on request through either intervention. Since patient navigation is a human-intensive intervention, examining the uptake of patient navigation when provided only on request is critical for conserving resources in limited-resource settings such as CHCs.
Participants
The study inclusion criteria aim to enrol a broad range of individuals to maximise reach. Eligible patients will be those who (1) have been seen at one of the participating CHCs in the last 3 years, (2) are 18 years and older, (3) have a working cellphone listed in the CHC EHR and (4) indicate a language preference in the EHR of English or Spanish.
The study will exclude participants who opt out on receipt of the introductory message asking about smartphone ownership. Also, if more than one patient shares the same smartphone number in the EHR, only the patient with the most recent documented clinical encounter will be included.
Recruitment
As a pragmatic trial with interventions that offer minimal risk, the University of Utah IRB approved a waiver of consent for randomisation and receipt of PHM interventions. Therefore, all participants who meet the eligibility criteria will be automatically enrolled in the study. All three study points of contact (TM, chatbot, PN) will allow participants to opt out through a simple reply at any time. Participants will be consented to complete the 3-month follow-up survey prior to survey completion using a consent cover letter.
Randomisation and blinding
Participants who self-report having a smartphone will be randomised to one of eight study arms (figure 1): (1) TM+10 day, (2) TM+30 day, (3) chatbot+10 day, (4) chatbot+30 day, (5) TM+PN+10 day, (6) TM+PN+30 day, (7) chatbot+PN+10 day or (8) chatbot+PN+30 day. Participants who do not self-report to have a smartphone will be randomised to one of four study arms: (1) TM+10 day, (2) TM+30 day, (3) TM+PN+10 day or (4) TM+PN+30 day. Participants will be randomised after receiving the introductory text message asking if they have access to a smartphone.
Figure 1.
SCALE-UP II trial design.
Randomisation will be implemented by software and will use randomised permuted blocks to guard against any biases due to the ordering of patients. Furthermore, the randomisation will be stratified by CHC and urban/rural designation of the participant’s zip code according to rural–urban commuting area codes.
The study is outcome assessor and investigator blinded. Patient navigators cannot be blinded to treatment assignment. Participants will be blinded to study participation.
Study interventions
Overall, all study interventions (1) are sent on behalf of the participant’s clinic, (2) offer the option to request at-home mailed COVID-19 test kits at no cost for use as needed, (3) are provided automatically in English or Spanish based on the patient’s preferred language in the CHC EHR and (4) provide an option for participants to opt-out at any time (see figures 2 and 3). Eligible patients and their demographic data (eg, name, date of birth, race, ethnicity, language, address, cellphone) will be extracted from the CHC EHRs through EHR reports prior to the trial launch. Demographic data will be used to determine eligibility, support study interventions and for study analyses.
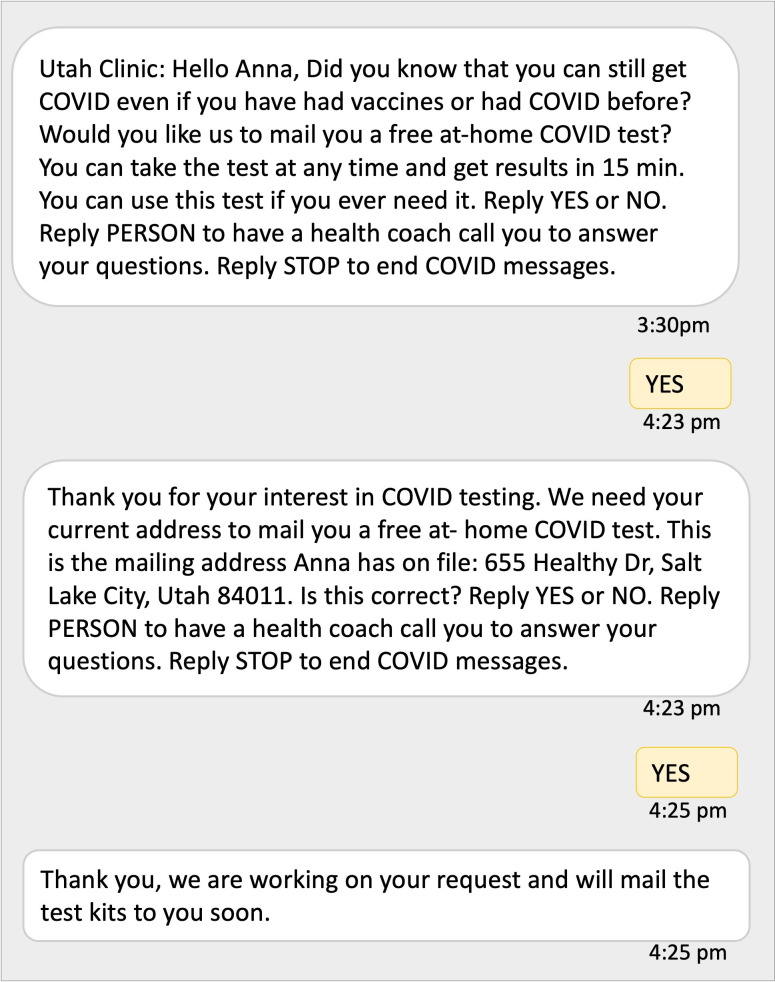
Figure 2.
Sample text message conversation offering COVID-19 at-home testing.
Figure 3.
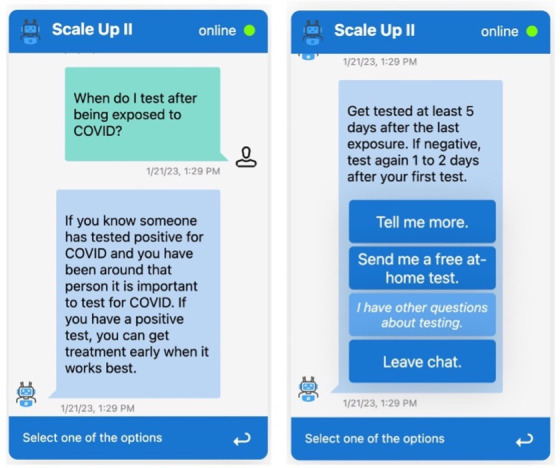
Sequential screenshot of chatbot intervention showing a question being answered, followed by options to ask further questions, and request a COVID-19 test kit to be mailed to the patient’s home.
Text messaging
Meta-analyses of TM interventions have found these approaches to be promising for improving compliance with healthy behaviours and preventive care, beneficial for multiple racial/ethnic groups and inexpensive to deliver.29–34 A US Department of Health and Human Services review concluded: With the near-ubiquitous presence of cellphones and the rapid growth of smartphones, TM and other mHealth interventions can remove traditional geographical and economic barriers to access to health information and services. The higher rates of mobile phone ownership and use among blacks and Hispanics, compared with whites, are particularly noteworthy. These interventions have the potential to improve health knowledge, behaviours and outcomes and, ultimately, to reduce disparities.34 Our own research has demonstrated that a simple, repeated offer to connect unmotivated, low-SES individuals with treatment resources resulted in 25% of individuals enrolling in tobacco cessation treatment.35 Thus, repeated prompting offering access to COVID-19 testing via texts is an extremely convenient, low-cost, scalable approach for increasing testing uptake.
TM will consist of bidirectional text messaging sent on behalf of the participant’s clinic with a response option for patients to request at-home mailed COVID-19 test kits at no cost (figure 2). Participants randomised to TM+PN will also be able to reply requesting to speak with a patient navigator. Messages will be sent in English or Spanish based on the patient’s language preference in the EHR.
Chatbot
Chatbots are automated conversational agents designed to mimic human interaction. Chatbots are increasingly popular in various health contexts as they can be easily accessed through smartphones, tablets, laptops or desktops. Chatbots have many advantages for patient engagement, including providing scripted education interactively, chunking information into small segments that are easier to process and allowing for choice in the amount of information received. Chatbots are accessible to the vast majority of US adults. Even in households with annual incomes less than US$30 000, 76% own a smartphone.16 36 37 Delivery of health services through chatbots in research contexts has been successfully tested in various health domains such as mental health, asthma, diabetes management and physical activity uptake.38 While scripted chatbots have been widely used in the COVID-19 pandemic, especially to evaluate patient’s eligibility for testing and vaccination,39–44 research is needed to examine their benefits in addressing COVID-19 at-home testing. In addition, there is a lack of studies that investigated the design and implementation of chatbots specifically for historically marginalised populations.
For SCALE-UP II, we designed a scripted chatbot (ie, predefined conversation script, and a fixed set of questions and scripted answers) that presents participants with a list of topics that address the most common knowledge gaps and hesitancy factors related to at-home COVID-19 testing (figure 3). The chatbot script was designed and guided by findings from a national survey and in-depth interviews with participants in the targeted Utah population, both conducted by our team. The following topics are covered: benefits of testing (even when already vaccinated or previously had COVID), when to test, test accuracy, how to use a test and what to do if a test is positive. Both text messaging and chatbot scripts were validated through feedback from the study and patient advisory committee composed of clinical staff and patients from the participating CHCs.
Patients in the chatbot condition will receive a text message on behalf of their clinic offering a hyperlink to access the chatbot on the phone’s web browser. At any point in the chatbot, participants will be able to click a button to request an at-home test kit. Participants randomised to the PN condition can also click PERSON to request to speak to a patient navigator. As in TM, the chatbot is offered both in English and Spanish.
Patient navigation
SCALE-UP II will use CHWs employed by AUCH as patient navigators to address practical barriers, motivation and hesitancy to COVID-19 testing. To assist navigators in working with patients, CHWs will be trained in an empirically validated behaviour change approach (Motivation And Problem Solving; MAPS).45–50 MAPS is a holistic, dynamic approach to behaviour change that integrates two empirically validated approaches (motivational interviewing51 52 and practical problem solving47 53 54) for helping patients engage in target behaviours.45–47 49 50 Importantly, MAPS addresses patients’ social determinants of health and provides practical advice and connections to services whenever possible, including addressing testing concerns (eg, worries about repercussions of a positive test, infecting family members, quarantining, financial). MAPS has been demonstrated to be effective in numerous randomised controlled trials with respect to increasing enrolment in evidence-based interventions, as well as enhancing and maintaining behaviour change.45–47 49 50
All SCALE-UP II navigators will receive ~20 hours of training, consistent with recommended training for helpline specialists.55 Participants randomised to the PN condition who request to speak with a patient navigator will receive a phone call within 48 hours, although it is anticipated that most patients will be called within the same day or the next day. Patient navigators will make three attempts to contact a participant.
Study roll-out schedule
To ensure that the interventions work properly with real patient data, a pilot study will be conducted with a random sample of patients from one of the participating CHCs both for the TM and chatbot interventions.
For the remainder of patients, to address bottlenecks that depend on non-automated processes (eg, mailing of test kits, patient navigation), study participants will be exposed to interventions in one of 14 weekly batches according to a predefined schedule, in which a cohort with a new set of participants is added to the study every week. Participants will be randomly allocated across the 14 batches, also stratified by CHC and urban vs rural.
Every cycle starts by sending an introductory message to participants in the cohort (Day −2). Participants will have 2 days to respond. After that, eligible participants will be randomised into one of the two factorial designs depending on their response to the introductory message (Day 0). After randomisation, participants will receive messages offering access to at-home testing once every 10 versus 30 days for 7 weeks (Days 0, 10, 20, 30, 40, 50 and 60 vs Days 0, 30 and 60).
Outcome assessment
The main study outcomes are described below. Table 1 provides a complete list including the primary, secondary outcomes and implementation outcomes, as well as predictors and moderators of study outcomes.
Table 1.
Study assessments
| Assessment | Baseline | During exposure to interventions | Day 90 follow-up (via text msg) | Day 97 follow-up (via survey) |
Description |
| Demographics | X | X | Age sex, race, ethnicity, preferred language, insurance status, etc. | ||
| Testing (primary outcome) | X | X | Proportion of study participants who use an at-home COVID-19 test during the course of the study. | ||
| Number of tests used | X | X | Self-reported number of tests used by each study participant who requested a test. | ||
| Vaccination status | X | X | COVID-19 vaccination status according to state immunisation registry | ||
| NIH RADx-UP CDE data elements (tier 1) | X | Comprehensive questionnaire (234 items) including demographics, COVID-19 testing, symptoms, health status, social determinants of health, etc. | |||
| Implementation outcomes | |||||
| Reach-engage testing | X | Proportion of participants who are offered at-home testing and reply to the message or launch the chatbot | |||
| Reach-engage frequency | X | Number of times a participant replied to a message offering at-home testing or launched the chatbot | |||
| Reach-accept testing | X | Proportion of participants who are offered at-home testing and reply accepting | |||
| Reach-engage frequency | X | Number of times a participant replied to a message/chatbot requesting at-home testing | |||
| PN-request | X | Proportion of participants in the PN condition who request patient navigation | |||
| PN-request frequency | X | Number of times a participant requested to speak with a patient navigator | |||
| PN-engage | X | Proportion of participants in the PN condition who talk to a patient navigator | |||
| PN-engage frequency | X | Number of times a participant spoke with a patient navigator | |||
| Opt-out | X | Proportion of participants who opted-out | |||
| Chatbot use | |||||
| Chatbot session length | X | Amount of time spent using the chatbot in a session | |||
| Chatbot timeout | X | Proportion of chatbot sessions that timed out without reaching an endpoint (eg, close chatbot window, request test, request to talk to patient navigator) | |||
| Chatbot actions | X | Number of chatbot topics clicked per session | |||
| Chatbot test request only | X | Proportion of chatbot session in which the only action was requesting a test | |||
| Chatbot coverage | X | Proportion of chatbot contents that are accessed per session | |||
| Chatbot topics | X | Proportion of sessions in which a specific chatbot topic is accessed | |||
CDE, Common Data Element; NIH, National Institutes of Health; PN, patient navigation; RADx-UP, Rapid Acceleration of Diagnostics-Underserved Populations.
Primary outcomes and hypotheses
The primary outcome is Testing; the proportion of study participants who use an at-home COVID-19 test at least once during the course of a 90-day study follow-up as defined below. For all patients, regardless of self-report of smartphone ownership, the primary hypotheses are main effects for PN (PN>No PN), main effects for message frequency (10-day>30-day) and that TM+PN will lead to higher Testing than TM. These hypotheses will be tested at an alpha of 0.0167, adjusted for multiple comparisons using the Bonferroni method. Because we anticipate a low sample size of smartphone self-reporters to be adequately powered, we consider chatbot-related hypotheses as secondary. These include the hypothesis that chatbot will lead to a higher Testing than TM and that chatbot+PN will lead to a higher Testing than chatbot without PN. These hypotheses will be tested at the alpha of 0.05.
Secondary and implementation outcomes
We will evaluate chatbot+PN versus chatbot, interaction effects and indicators of TM, chatbot and PN implementation among participants. Implementation outcomes measure the extent of the delivery and adaptation of intervention components, including Reach-Engage Testing (proportion of participants who are offered at-home testing and reply to the message or launch the chatbot), Reach-Accept Testing (proportion of participants who are offered at-home testing and reply accepting or select ‘Send me a test’ on the chatbot), PN-Request (proportion of participants in the PN condition who request patient navigation), PN-Engage (proportion of participants in the PN condition who talk to a patient navigator) and Opt-Out (proportion of participants who opt-out). We will analyse chatbot usage patterns (eg, time using chatbot, topics visited) as listed in table 1.
Predictors and moderators of study outcomes
We will assess predictors and moderators including demographics, vaccination status and tier 1 Common Data Elements (CDEs) used by the Rapid Acceleration of Diagnostics-Underserved Populations programme of the US National Institutes of Health.56
Study assessments
The primary outcome Testing will be collected through two methods from patients who requested a test kit: (1) a brief text message sent 90 days after the first exposure to interventions asking if they used the mailed COVID-19 test at least once (patients are asked to reply with a single YES or NO response to the text message); and (2) a survey sent to participants 7 days after the last exposure to study interventions (Day 97). Secondary outcomes Reach-Engage Testing, Reach-Accept Testing, PN-Request, PN-Engage and Opt-Out will be obtained from computer system logs. The survey will also collect tier 1 CDEs. To complete the survey, participants will be invited via mail and text message to complete a survey. Non-responders will also be called via phone to complete an interviewer-administered survey. Vaccination status will be obtained from the Utah State Immunization Information System. Other predictors and moderators of study outcomes will be collected from EHR data (eg, demographics) and online surveys (ie, tier 1 CDEs).
Statistical analysis
The main effects of each intervention will be evaluated using a logistic regression model by regressing 90-day testing on each of the three main effects: Chatbot (vs TM) with an indicator for self-reporting to have a smartphone, PN (vs no PN) and outreach frequency (10 vs 30 days). We will preliminarily include the pairwise interactions of the main effects, and the three-way interaction to assess for any synergistic and/or antagonistic effect modifications across interventions and will include any statistically significant effect modifications (ie, interactions) in the primary analysis model. The model will adjust for whether the patient self-reported having a smartphone. Estimates and 95% CIs will be reported for each main effect and interaction effect. If an interaction term was included for having evidence of an effect modification, we will report the main effects separately for each level of the effect-modifying intervention. The model will be run on all participants to evaluate the primary hypotheses, each tested at the alpha of 0.0167, and it will be applied to the smartphone participants to evaluate the secondary hypotheses.
Among the smartphone subgroups, we will fit the primary analysis model to evaluate all other main effects as a secondary analysis. We will also test the added effect of PN among those randomised to receive chatbot. Among the remaining patients, we will regress 90-day testing on PN (yes vs no) and outreach frequency (10 vs 30 days). We will include an interaction if a preliminary model provides evidence of an effect modification. Side-by-side, we will present the estimated effects across all patients and by smartphone ownership subgroup.
Handling missing data
The primary analysis will assume missing outcomes and covariates are missing at random (MAR). Under this assumption, observed covariates can be used to explain the missingness mechanism. When conditioning on observed covariates, the distribution of outcomes is assumed to be similar among responders and non-responders. With this framework, we will omit missing outcomes,57 multiply impute missing covariates using a fully conditional specified model58 and account for the multiple imputations in analysis.59 While MAR is considered a reasonable starting point assumption for missing data, it is plausible that responders and non-responders have different outcomes beyond what can be adjusted by covariates (ie, missing not at random). We will use pattern mixture models as a sensitivity analysis to assess the robustness of conclusions under the MAR assumption.60
Sample size justification
Power for SCALE-UP II was evaluated for a target enrolment of 42 000 adults aged 18 years and older who receive care at the three participating CHCs, have a valid cellphone recorded in the EHR and have English or Spanish as their preferred language in the EHR. This estimate is based on the patient population that has received care at the three participating CHCs within the 3 years preceding the trial and who meet the inclusion criteria. Among those patients, we anticipate fewer than 10% opt-outs based on prior studies using similar population health management approaches with the same CHCs.61 Based on national estimates of smartphone ownership,62 we assume 75% will have a smartphone and 10% to self-report as having a smartphone. Among these patients with a self-reported smartphone, we anticipate ~375 patients in each of the eight study arms. Among patients who do not self-report as having a smartphone, we expect ~8750 patients in each of the four arms.
Based on the results of our previous trial using text messaging to help patients with access to COVID-19 testing,61 we estimate that TM with no PN and a 30-day outreach will have a 5% at-home testing rate and that PN, chatbot and 10-day outreach frequency will increase the testing rate by 5% each without a synergistic effect. We hypothesise the at-home testing rate to be 5% less when outreach occurs every 30 days. Based on a similar trial conducted with patients from the same CHCs, we anticipate a >40% response rate for the primary outcome.63 Under these assumptions, with alpha adjusted to 0.0167, and assuming the response rate is 20%, we are at least 85% powered to test these effects. In secondary analyses, with alpha of 0.05 and a 40% response rate, we are 75% powered to detect the chatbot main effect of and 68% powered to detect the added effect of PN.
Ethics and dissemination
All procedures performed in studies involving human participants will be conducted in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The protocol for this study was approved by the University of Utah IRB (00150669). Materials used to conduct the study are not currently publicly available. Materials may be requested by emailing the corresponding author. Study results will be disseminated via peer-reviewed publications and manuscripts, as well as to the health system and community partners via lay reports and presentations.
Discussion
Individuals from historically marginalised communities have suffered substantial health inequities throughout the COVID-19 pandemic, not only in terms of outcomes but also vaccination rates and access to testing.1–8 64 65 PHM approaches leveraging widely adopted EHR systems and technology such as cellphones provide excellent opportunities to deliver scalable interventions to improve health equity. The SCALE-UP II trial aims to examine scalable and sustainable PHM interventions to increase the uptake of at-home COVID-19 testing among individuals who receive care from low-resource CHCs. Strengths include a pragmatic trial with broad inclusion criteria leveraging existing EHR data; highly scalable automated interventions; and a novel design that compares two digital patient engagement approaches (TM and chatbot), examines the added effect of a human-augmented intervention (patient navigation) over digital interventions, and compares two frequencies (every 10 days or 30 days) of repeated offers to receive COVID-19 testing.
Even though public health agencies worldwide have declared the end of the COVID-19 pandemic, COVID-19 testing is still critical to help reduce exposure and identify individuals who can benefit from treatment. In addition, approaches are needed to support public health preparedness for future pandemics and outbreaks. The proposed interventions in SCALE-UP II leverage resources that are currently available at CHCs and therefore can be sustained in the long term.
Limitations
The study design has several limitations. First, a potentially low response rate to the introductory message asking about smartphone ownership could lead to small sample size and randomisation of only motivated individuals to the chatbot condition. We considered randomising all participants to chatbot versus TM, but patients who do not have a smartphone (estimated as 25% of the CHC patient population) and are randomised to the chatbot condition would not be able to use the chatbot, compromising study reach. Second, the study relies on self-report for the primary outcome with a 90-day follow-up interval (Testing). Patients may not recall test use and may be less likely to self-report test use after 90 days of requesting a test. However, a 90-day follow-up was chosen to give participants sufficient time to actually use a kit, given that participants could request a test kit regardless of current symptoms and/or exposure and use the test whenever needed. To maximise response rates, we use two approaches to collect the primary outcome: a quick question via text messaging 90 days after exposure to study interventions and a survey at the end of the study, using multiple contact attempts as well as pre-participation and post-participation incentives.
Last, the study will be conducted after the peak of the pandemic, when participants may be less motivated to learn about and receive COVID-19 testing. Also, individuals have been overexposed to information about COVID-19 from multiple sources and may have already formed their opinions about COVID-19 and COVID-19 testing. Therefore, it is possible that study findings may not generalise to the context of the new onset of a pandemic or outbreak.
Supplementary Material
Footnotes
Contributors: GDF, BO, TVK, JC, TG, KKK, KK, CYL, CRS and DWW provided substantial contributions to the conception of the study. All coauthors provided substantial contributions to the study design. GDF, BO, TVK and JC drafted the manuscript. All coauthors reviewed the manuscript critically for important intellectual content. All coauthors provided final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding: This work was supported by the National Institute on Minority Health and Health Disparities (NIMHHD) of the US National Institutes of Health (NIH) grant number 5U01MD017421.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1. Adhikari S, Pantaleo NP, Feldman JM, et al. Assessment of community-level disparities in Coronavirus disease 2019 (COVID-19) infections and deaths in large US metropolitan areas. JAMA Netw Open 2020;3:e2016938. 10.1001/jamanetworkopen.2020.16938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol 2020;47:37–44. 10.1016/j.annepidem.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA 2020;323:2466–7. 10.1001/jama.2020.8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Romano SD, Blackstock AJ, Taylor EV, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region - United States, March-December 2020. MMWR Morb Mortal Wkly Rep 2021;70:560–5. 10.15585/mmwr.mm7015e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murthy BP, Sterrett N, Weller D, et al. Disparities in COVID-19 vaccination coverage between urban and rural counties—United States. MMWR Morb Mortal Wkly Rep 2020;70:759–64. 10.15585/mmwr.mm7020e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barry V, Dasgupta S, Weller DL, et al. Patterns in COVID-19 vaccination coverage, by social vulnerability and Urbanicity—United States. MMWR Morb Mortal Wkly Rep 2020;70:818–24. 10.15585/mmwr.mm7022e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reitsma MB, Goldhaber-Fiebert JD, Salomon JA. Quantifying and Benchmarking disparities in COVID-19 vaccination rates by race and Ethnicity. JAMA Netw Open 2021;4:e2130343. 10.1001/jamanetworkopen.2021.30343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams AM, Clayton HB, Singleton JA. Racial and ethnic disparities in COVID-19 vaccination coverage: the contribution of socioeconomic and demographic factors. Am J Prev Med 2022;62:473–82. 10.1016/j.amepre.2021.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. ABC4 . COVID-19 testing site at Maverik center closing. Retreived from ABC4.com. n.d. Available: https://www.abc4.com/coronavirus/covid-19-testing-site-at-maverik-center-closing/ [Google Scholar]
- 10. Herald M. Florida-run COVID testing sites closing at the end of May. Where can you go next? Retrieved from Miami Herald website. n.d. Available: https://www.miamiherald.com/news/coronavirus/article251528343.html [Google Scholar]
- 11. Mercer TR, Salit M. Testing at scale during the COVID-19 pandemic. Nat Rev Genet 2021;22:415–26. 10.1038/s41576-021-00360-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubin R. COVID-19 testing moves out of the clinic and into the home. JAMA 2021;326:1362. 10.1001/jama.2021.15679 [DOI] [PubMed] [Google Scholar]
- 13. Rader B, Gertz A, Iuliano AD, et al. Use of at-home COVID-19 tests - United States. MMWR Morb Mortal Wkly Rep 2022;71:489–94. 10.15585/mmwr.mm7113e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adler-Milstein J, Holmgren AJ, Kralovec P, et al. “Electronic health record adoption in US hospitals: the emergence of a Digital “advanced use” divide”. J Am Med Inform Assoc 2017;24:1142–8. 10.1093/jamia/ocx080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kruse CS, Kristof C, Jones B, et al. Barriers to electronic health record adoption: a systematic literature review. J Med Syst 2016;40:252. 10.1007/s10916-016-0628-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. PEW Research Center . Mobile fact sheet. n.d. Available: https://www.pewresearch.org/internet/fact-sheet/mobile/
- 17. Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schlechter CR, Del Fiol G, Lam CY, et al. Application of community - engaged dissemination and implementation science to improve health equity. Prev Med Rep 2021;24:101620. 10.1016/j.pmedr.2021.101620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schlechter CR, Reese TJ, Wirth J, et al. Rapid-cycle designs to adapt interventions for COVID-19 in safety-Net Healthcare systems. Transl Behav Med 2023;13:389–99. 10.1093/tbm/ibac101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henry J, Pylypchuk Y, Searcy T, et al. Adoption of electronic health record systems among US non-Federal acute care hospitals: 2008-2015. ONC Data Brief 2016;35:1–9. [Google Scholar]
- 21. Office-based physician electronic health record adoption. 2021. Available: https://www.healthit.gov/data/quickstats/office-based-physician-electronic-health-record-adoption
- 22. National center for health statistics. ambulatory care use and physician office visit. 2018. Available: https://www.cdc.gov/nchs/fastats/physician-visits.htm
- 23. UDOH . Behavioral risk factor surveillance system (BRFSS) data on obesity. Retrieved from Utah Department of Health, Center for Health Data and Informatics, Indicator-Based Information System for Public Health website. n.d. Available: http://ibis.health.utah.gov/ [Google Scholar]
- 24. Ali MK, McKeever Bullard K, Imperatore G, et al. Reach and use of diabetes prevention services in the United States, 2016-2017. JAMA Netw Open 2019;2:e193160. 10.1001/jamanetworkopen.2019.3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ariel-Donges AH, Gordon EL, Dixon BN, et al. Rural/urban disparities in access to the National diabetes prevention program [Translational behavioral medicine]. Transl Behav Med 2020;10:1554–8. 10.1093/tbm/ibz098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McBrien KA, Ivers N, Barnieh L, et al. Patient navigators for people with chronic disease: A systematic review. PLoS One 2018;13:e0191980. 10.1371/journal.pone.0191980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peart A, Lewis V, Brown T, et al. Patient navigators facilitating access to primary care: a Scoping review. BMJ Open 2018;8:e019252. 10.1136/bmjopen-2017-019252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Valaitis RK, Carter N, Lam A, et al. Implementation and maintenance of patient navigation programs linking primary care with community-based health and social services: a Scoping literature review. BMC Health Serv Res 2017;17:116. 10.1186/s12913-017-2046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cole-Lewis H, Kershaw T. Text Messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev 2010;32:56–69. 10.1093/epirev/mxq004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Car J, Gurol-Urganci I, de Jongh T, et al. Mobile phone Messaging reminders for attendance at Healthcare appointments. Cochrane Database Syst Rev 2012;2013:CD007458. 10.1002/14651858.CD007458.pub2 [DOI] [PubMed] [Google Scholar]
- 31. Head KJ, Noar SM, Iannarino NT, et al. Efficacy of text Messaging-based interventions for health promotion: a meta-analysis. Social Science & Medicine 2013;97:41–8. 10.1016/j.socscimed.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 32. Schwebel FJ, Larimer ME. Using text message reminders in health care services: A narrative literature review. Internet Interv 2018;13:82–104. 10.1016/j.invent.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vodopivec-Jamsek V, de Jongh T, Gurol-Urganci I, et al. Mobile phone Messaging for preventive health care. Cochrane Libr 2012. 10.1002/14651858.CD007457.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. USDHHS . Using Health Text Messages To Improve Consumer Health Knowledge, Behaviors, And Outcomes: An Environmental Scan. Rockville, MD: US Department of Health and Human Services, 2014. [Google Scholar]
- 35. Wetter DW. How can we help more Smokers engage in evidence-based treatment? Annual Conference of the Utah Cancer Action Network; Salt Lake City, UT2017, [Google Scholar]
- 36. Anderson M. Mobile technology and home Broadband. n.d. Available: https://www.pewresearch.org/internet/2019/06/13/mobile-technology-and-home-broadband-2019/
- 37. Center PR. Internet/Broadband fact sheet. n.d. Available: https://www.pewresearch.org/internet/fact-sheet/internet-broadband/
- 38. Kocaballi AB, Berkovsky S, Quiroz JC, et al. The Personalization of conversational agents in health care. J Med Internet Res 2019;21:e15360. 10.2196/15360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Judson TJ, Odisho AY, Neinstein AB, et al. Rapid design and implementation of an integrated patient self-triage and self-scheduling tool for COVID-19. J Am Med Inform Assoc 2020;27:860–6. 10.1093/jamia/ocaa051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Judson TJ, Odisho AY, Young JJ, et al. Implementation of a Digital Chatbot to screen health system employees during the COVID-19 pandemic. J Am Med Inform Assoc 2020;27:1450–5. 10.1093/jamia/ocaa130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McKillop M, South BR, Preininger A, et al. Leveraging conversational technology to answer common COVID-19 questions. J Am Med Inform Assoc 2021;28:850–5. 10.1093/jamia/ocaa316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dennis AR, Kim A, Rahimi M, et al. User reactions to COVID-19 screening Chatbots from reputable providers. J Am Med Inform Assoc 2020;27:1727–31. 10.1093/jamia/ocaa167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ford D, Harvey JB, McElligott J, et al. Leveraging health system Telehealth and Informatics infrastructure to create a continuum of services for COVID-19 screening, testing, and treatment. J Am Med Inform Assoc 2020;27:1871–7. 10.1093/jamia/ocaa157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martin A, Nateqi J, Gruarin S, et al. An artificial intelligence-based first-line defence against COVID-19: Digitally screening citizens for risks via a Chatbot. Sci Rep 2020;10:19012. 10.1038/s41598-020-75912-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Correa-Fernández V, Díaz-Toro EC, Reitzel LR, et al. Combined treatment for at-risk drinking and smoking cessation among Puerto Ricans: a randomized clinical trial. Addict Behav 2017;65:185–92. 10.1016/j.addbeh.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McClure JB, Westbrook E, Curry SJ, et al. Proactive, Motivationally enhanced smoking cessation counseling among women with elevated Cervical cancer risk. Nicotine & Tobacco Research 2005;7:881–9. 10.1080/14622200500266080 [DOI] [PubMed] [Google Scholar]
- 47. Reitzel LR, Vidrine JI, Businelle MS, et al. Preventing postpartum smoking relapse among diverse low-income women: a randomized clinical trial. Nicotine Tob Res 2010;12:326–35. 10.1093/ntr/ntq001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vidrine JI, Reitzel LR, Figueroa PY, et al. Motivation and problem solving (MAPS): Motivationally-based skills training for treating substance use. Cogn Behav Pract 2013;20:501–16. 10.1016/j.cbpra.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vinci C, Lam CY, Schlechter CR, et al. Increasing treatment engagement among Smokers who are not motivated to quit: A randomized clinical trial. [DOI] [PMC free article] [PubMed]
- 50. Wetter DW, Mazas C, Daza P, et al. Reaching and treating Spanish-speaking Smokers through the National Cancer Institute’s cancer information service. a randomized controlled trial. Cancer 2007;109(2 Suppl):406–13. 10.1002/cncr.22360 [DOI] [PubMed] [Google Scholar]
- 51. Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York: The Guildford Press, 1991. [Google Scholar]
- 52. Miller WR, Rollnick S. Motivational Interviewing. 2nd edition. New York: The Guilford Press, 2002. [Google Scholar]
- 53. Marlatt G, Donovan D. Relapse prevention: Maintenance Strategies in the Treatment of Addictive Behaviors 2nd edition. New York: Guilford Press, 2005. [Google Scholar]
- 54. Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. Am Psychol 2004;59:224–35. 10.1037/0003-066X.59.4.224 [DOI] [PubMed] [Google Scholar]
- 55. CDC . Telephone Quitlines: A resource for development, implementation, and evaluation. In: Office of Smoking and Health, Final Edition. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, 2004. [Google Scholar]
- 56. Rubinstein YR, McInnes P. NIH/NCATS/GRDR® common data elements: a leading force for standardized data collection. Contemp Clin Trials 2015;42:78–80. 10.1016/j.cct.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Little RJ, Rubin DB. Statistical analysis with missing data, third edition. In: Statistical analysis with missing data. John Wiley & Sons, 17 April 2019. 10.1002/9781119482260 [DOI] [Google Scholar]
- 58. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–42. 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 59. Rubin DB. Multiple imputation for Nonresponse in surveys. In: DB: Multiple imputation for nonresponse in surveys: Wiley XXIX+. New York: Springer, June 9, 1987: 10.1002/9780470316696 [DOI] [Google Scholar]
- 60. Cro S, Morris TP, Kenward MG, et al. Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: A practical guide. Stat Med 2020;39:2815–42. 10.1002/sim.8569 [DOI] [PubMed] [Google Scholar]
- 61. Schlechter CR, Del Fiol G, Wetter DW, eds. Rapid and responsive interventions to address COVID-19 and promote health equity. In: 15th Annual Conference of Dissemination and Implementation in Health 2022. Washington, DC: AcademyHealth, [Google Scholar]
- 62. Center PR. Mobile fact sheet. 2021. Available: https://www.pewresearch.org/internet/fact-sheet/mobile/
- 63. Fernandez ME, Schlechter CR, Del Fiol G, et al. Quitsmart Utah: an implementation study protocol for a cluster-randomized, multi-level sequential multiple assignment randomized trial to increase reach and impact of tobacco cessation treatment in community health centers. Implement Sci 2020;15:9. 10.1186/s13012-020-0967-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Khullar D, Zhang Y, Zang C, et al. Racial/ethnic disparities in post-acute sequelae of SARS-Cov-2 infection in New York: an EHR-based cohort study from the RECOVER program. J Gen Intern Med 2023;38:1127–36. 10.1007/s11606-022-07997-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pfaff ER, Madlock-Brown C, Baratta JM, et al. Coding long COVID: characterizing a new disease through an ICD-10 lens. BMC Med 2023;21:58. 10.1186/s12916-023-02737-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.