Abstract
Background:
Cognitive health is a public health concern among older adults. Dietary supplement (SUP) use is common and concerns have been raised about high folic acid intake among those with vitamin B-12 deficiency and exacerbation of poor cognitive performance (PCP).
Objectives:
We evaluated SUP use, usual folic acid intake, and blood folate and vitamin B-12 concentrations in relation to cognitive performance.
Methods:
We used NHANES 2011–2014 data on adults aged ≥60 y (n = 2867) and estimated total usual folic acid intake from diet and supplements, vitamin B-12 intake from SUPs, blood folates, vitamin B-12 concentrations, vitamin B-12 insufficiency (≤258 pmol/L), high folate (serum folate ≥59 nmol/L or RBC folate ≥1609 nmol/L), and PCP (<34 on the Digit Symbol Substitution Test). We assessed folate distributions adjusted for multiple variables, including renal function.
Results:
Compared with persons without PCP, adults with PCP were less likely to use supplements containing folic acid (mean ± SEE: 34.4% ± 2.4%) or vitamin B-12 (mean ± SEE: 47.5% ± 1.6%). Among vitamin B-12–insufficient adults, 18.0% ± 1.6% (mean ± SEE) reported taking a vitamin B-12 supplement. Among participants with high folate and insufficient vitamin B-12 concentrations, 34.3% ± 11.5% (mean ± SEE) reported taking vitamin B-12–containing supplements. Persons with high folate and normal vitamin B-12 concentrations had lower odds of PCP [aOR (adjusted odds ratio): 0.61; 95% CI: 0.45, 0.83] than persons with normal folate and vitamin B-12. Persons with high folate and normal methylmalonic acid (MMA) had lower odds of PCP (OR: 0.56; 95% CI: 0.40, 0.78) than those with normal folate and MMA concentrations. After adjustment for renal function, elevated risk of PCP was attenuated among persons with high folate and MMA. Concurrent high folate and insufficient vitamin B-12 concentrations were not associated with PCP.
Conclusions:
Differential associations between vitamin B-12 and MMA highlight the need to consider renal function in studies of high folate and low vitamin B-12 status. Consumption of vitamin B-12 supplements concurrent with low vitamin B-12 status may indicate vitamin B-12 malabsorption. Am J Clin Nutr 2022;116:74–85.
Keywords: folic acid, vitamin B-12, kidney dysfunction, poor cognitive performance, supplements, malabsorption, older adults
Introduction
Cognitive health is an important public health concern among older adults and may be influenced by genetic (e.g., APOE genotype), environmental (e.g., ambient air pollution), as well as health or lifestyle factors (e.g., smoking) (1-4). Deterioration in cognitive functioning can negatively influence personal relationships, quality of life, and independence and can lead to additional health care needs and caregiving and financial challenges (5, 6). Patients with symptoms of poor cognition often delay necessary and appropriate clinical evaluation (7). The estimated prevalence of cognitive impairment among older adults ranges from 3% to 24% owing to inconsistent classification tools and definitions being used (8, 9).
The use of dietary supplements (SUPs) is becoming increasingly prevalent among older adults owing to their perceived benefits for cognition and overall health (10-12). Of adults aged ≥60 y, >70% reported using an SUP daily and 52% reported using a vitamin B-12–containing SUP (17). However, despite the ubiquitous use of vitamin B-12 supplements, older adults can remain vitamin B-12 insufficient because of age, diet, and certain medical conditions (e.g., gastrointestinal disorders, pernicious anemia) that result in vitamin B-12 malabsorption (13-17). Folate is a B vitamin important in basic cell processes and commonly available as folic acid in supplements and food fortification. Previous studies have raised a concern that “high” folate intake among older adults with low vitamin B-12 status may exacerbate poor cognitive performance (PCP) (18, 19). Currently, no formal definition of “high” folate exists because it is not associated with adverse health outcomes and because cutoffs are arbitrary and/or inconsistently used in the literature.
Folate and vitamin B-12 have overlapping and unique manifestations of deficiency. Severe deficiency in either folate or vitamin B-12 results in megaloblastic anemia, due to the inhibition of cell division and disrupted DNA replication [reviewed in Berry (20)]. Unlike natural food folate, folic acid can rescue DNA replication and correct the anemia without the presence of vitamin B-12. However, folic acid is unable to prevent or treat the neurologic problems associated with vitamin B-12 deficiency. If left untreated, vitamin B-12 deficiency can lead to irreversible neurologic damage. Historical case reports suggested that cognitive issues persisted in persons despite receiving treatment of pernicious anemia with folic acid [reviewed in Berry (20)]. This led to concerns about the exposure of folic acid through food fortification or supplement use and its effects (i.e., masking) on those with vitamin B-12 deficiency (after vitamin B-12 was discovered and characterized) [reviewed in Berry (20)]. These concerns were alleviated with the availability of clinical vitamin B-12 testing [reviewed in Berry (20)].
In 1998, the United States began mandatory folic acid fortification of enriched cereal-grain products (ECGPs) for the prevention of neural tube defects (NTDs) (21), which has since averted ≥1300 NTDs annually and largely prevented folate deficiency and its related anemia across the US population (22, 23). Studies have reported that in the absence of vitamin B-12 deficiency, “higher” folate concentrations were not associated with poor cognition among older adults (18, 19, 24). However, concerns remain over the interrelation between folate and vitamin B-12 as it relates to cognitive performance (25). Therefore, we conducted a cross-sectional study in adults ≥60 y old to evaluate SUP use and usual folic acid intake, as well as vitamin B-12, methylmalonic acid (MMA), and blood folate concentrations, in the context of cognitive performance.
Methods
Demographics
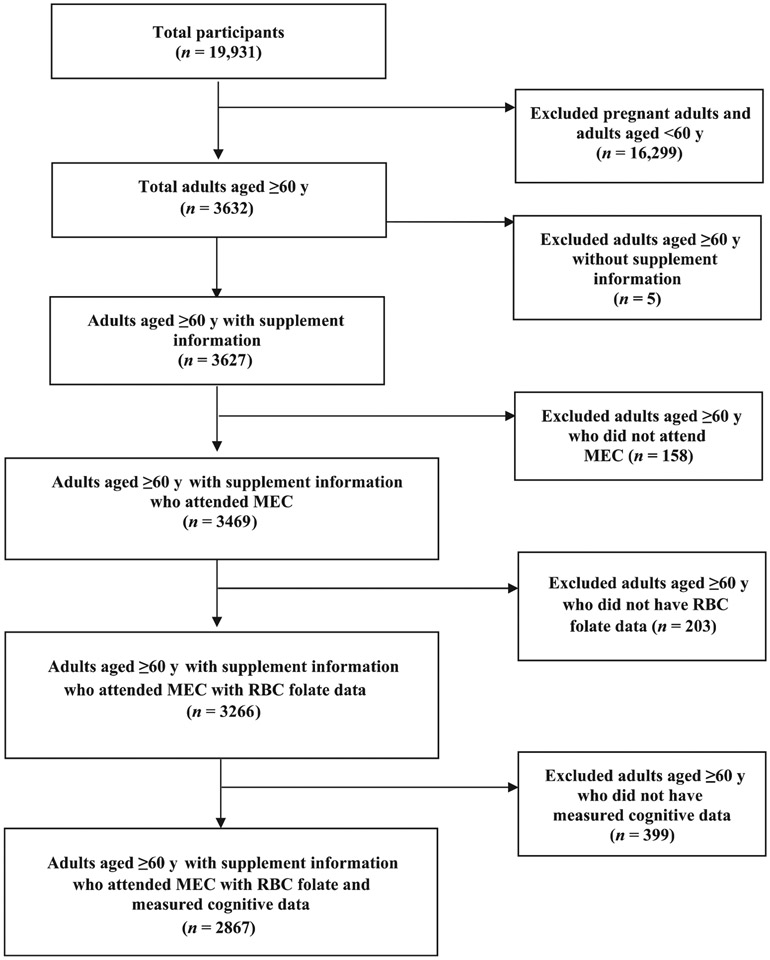
We combined 2011–2012 and 2013–2014 NHANES data into a single data set (2011–2014) to describe the demographic characteristics of our population, to assess folic acid intake (usual and from SUPs only), vitamin B-12 intake from SUPs, and to calculate folate and vitamin B-12 concentrations among adults aged ≥60 y. NHANES is a cross-sectional survey conducted in 2-y phases using a stratified multistage probability design to capture a nationally representative sample of the noninstitutionalized civilian US population. The survey design and procedures have been described in detail elsewhere (26). Briefly, trained interviewers gather health information biennially from a sample of adults using phone and in-home interviews, followed by a physical examination in a mobile examination center (MEC). The protocols for conducting NHANES were approved by the institutional review board of the National Center for Health Statistics, CDC, and informed consent was obtained from all participants. Adults aged ≥60 y with supplement use information who attended the MEC examination and had an RBC folate concentration measurement and cognitive performance data were included in the analyses (n = 2867) (Figure 1).
FIGURE 1.
Participant flowchart NHANES 2011–2014. MEC, mobile examination center.
Cognitive assessment
The Digit Symbol Substitution Test (DSST) is a performance module from the Wechsler Adult Intelligence Scale, Third Edition (WAIS III) that primarily assesses processing speed, sustained attention, and working memory (27). The DSST has been widely used to assess aspects of cognitive functioning. The test was administered in paper form to adults aged ≥60 y. Using a key containing 9 numbers paired with symbols at the top of the paper form, participants had 2 min to copy the corresponding symbols in the 133 boxes that connected the numbers. The score was the total number of correct matches achieved within the allotted time. Higher scores were indicators of better cognitive performance. We used DSST <34—the 25th percentile of the distribution—as a cutoff in NHANES to classify PCP for adults aged ≥60 y based on the methods used in the published literature (24, 28). As a sensitivity analysis, we reran our analyses with other measures of cognitive performance available through NHANES 2011–2014, including word recall tests from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) testing battery and an Animal Fluency (AF) test, using the same exposure percentile cutoffs for each of these analyses. CERAD is used to identify Alzheimer’s disease and consists of a 10-item word list learning with both immediate [CERAD-WL (Consortium to Establish a Registry for Alzheimer’s Disease—Word List Memory Task)] and delayed recall [CERAD-DR (Word List Recall Test)]. AF measures verbal fluency and asks participants to name as many animals as possible in 1 min. These additional cognitive tests include executive function and a memory subdomain that assesses the ability to learn new verbal information (29). Based on the literature, the following cutoffs were used to indicate PCP for these additional cognitive tests: <17 for CERAD-WL, <5 for CERAD-DR, and < 14 for AF (29, 30).
SUP use and usual folic acid intake
SUP use was ascertained by asking participants to recall their supplement use in the past 30 d. Intakes of foods in the previous 24 h were collected in person in the MEC (day 1) and by telephone 3–10 d later (day 2). Supplement use data were used to define the types and amounts of SUPs consumed to estimate intakes of nutrients from those SUPs. Participants who reported supplement use were asked to provide the name of the supplement, frequency of use, and typical dose. We extracted information related to folic acid and vitamin B-12 supplement use. These supplements were reported as microgram (μg) dosage during the past 30 d. To estimate usual intake of folic acid, we used the National Cancer Institute macro method, which took age, gender, poverty:income ratio (PIR), and race/ethnicity into account as covariates (31). Total usual intake distributions of folate were estimated using available nutrient components (31). We categorized usual daily intake of folic acid into ≥400 μg or ≥1000 μg [the tolerable upper intake level (UL)] (21).
Vitamin B-12, MMA, and folate status
Blood draws were conducted in an MEC and NHANES serum specimens were processed, stored, and shipped to the Division of Laboratory Sciences, National Center for Environmental Health, CDC for analysis. Serum vitamin B-12 was measured using the Elecsys Vitamin B-12 assay, which uses a competitive electrochemiluminescence immunoassay using intrinsic factor specific for vitamin B-12. Serum vitamin B-12 insufficiency was defined as serum vitamin B-12 ≤258 pmol/L (32-34). Serum MMA was measured using LC–MS and liquid chromatography–tandem mass spectrometry (LC–MS/MS). Elevated MMA was defined as >260 nmol/L and sensitivity analyses were performed for MMA ≥210 nmol/L (33, 35, 36). MMA was independently assessed because it is highly specific to vitamin B-12 deficiency and is not easily influenced by vitamin B-6 or folate concentrations (37, 38).
Folate status was ascertained using serum and RBC folate concentrations, which were measured in NHANES using the LC–MS/MS and microbiological assay methods, respectively, and log transformed before analysis (39). High folate status was classified in various ways because there is no consistent cutoff used in the literature. We assessed serum and RBC folate among a series of percentile cutoffs (25th, 50th, 75th, 90th, 95th, and 97th percentiles). Based on previous publication findings, we also assessed folate status using a combination of serum and RBC concentrations (59 nmol/L and 1609 nmol/L, respectively) (18, 19).
Study variables
Participants were categorized by age (60–64, 65–69, 70–74, 75–79, ≥80 y), gender (female/male), race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, other), education [<high school, high school graduate or equivalent (General Educational Development), >high school], marital status (married/living together, widowed/divorced/separated, never married), alcohol use status (<1, 1 to <2, ≥2 drinks/d), and PIR (<1, 1 to <2, 2 to <4, ≥4). PIR is the total household income divided by the poverty threshold according to the US Census Bureau and accounting for family size, year, and state (26). A PIR <1 reflects adults living below the poverty level.
Participant height and weight were measured during MEC examinations and were used to calculate the BMI (in kg/m2). We categorized BMI into <25, 25 to <30, and ≥30. Smoking status (nonsmoker, smoker) was determined using serum cotinine concentrations from samples taken at the MEC (cotinine concentrations >10 ng/mL were classified as smokers) (37).
In the United States there are 3 main sources of folic acid: ECGPs, ready-to–eat cereals (RTEs), and SUPs. ECGPs are grain products labeled “enriched” and are required to be fortified with 140 μg folic acid per 100 g of product. RTEs are voluntarily fortified and can contain ≤400 μg folic acid per serving (21). SUPs typically contain 400–800 μg folic acid. We created 4 mutually exclusive folic acid intake groups: those who reported consuming 1) folic acid from ECGPs only (ECGPs), 2) ECGPs and RTEs that contained folic acid (ECGPs + RTEs), 3) ECGPs and SUPs that contained folic acid (ECGPs + SUPs), and 4) all 3 sources (ECGPs + RTEs + SUPs). We categorized the average intake per day of folic acid supplement use as nonusers, <400 μg, 400 to <800 μg, 800 to ≥1000 μg, and ≥1000 μg and vitamin B-12 supplement use as nonusers, ≤6 μg, >6 to 25 μg, and >25 μg.
We used the following measures to assess kidney function: serum creatinine, albumin:creatinine ratio (ACR), and estimated glomerular filtration rate (eGFR). Serum creatinine was defined as high (≥1.3 mg/dL) and not high (<1.3 mg/dL). ACR (mg/g) was calculated as urine albumin (mg/dL) divided by urine creatinine (g/dL), and categorized into <30 mg/g, 30–300 mg/g, and >300 mg/g (elevated ACR). eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for calibrated creatinine and classified as reduced kidney function if values ranged from 0 to <60 mL · min−1 · 1.73 m−2 (40).
Participants also were asked whether they had ever been diagnosed with medical conditions (e.g., cancer, diabetes) and self-reported responses were obtained. Fasting status was defined as no food or supplement use for ≥8 h before the blood draw. We analyzed unmetabolized folic acid (UMFA) as a categoric variable at the 80th percentile cutoff of the distribution (≥2 or <2 nmol/L) (41, 42).
Statistical analysis
We used NHANES 4-y MEC sampling weights to analyze the survey data and account for nonresponse, unequal probabilities of selection, and poststratification. Chi-square tests and t tests were used to assess statistical differences; we considered P values < 0.05 to be statistically significant. Logistic regression univariable and multivariable models were developed to estimate the ORs and 95% CIs. The multivariable models were developed using an iterative stepwise procedure to identify factors potentially associated with PCP. We adjusted for model 1 (age, gender, ethnicity, marital status, education, smoking, PIR, eGFR, and ACR); model 1 + vitamin B-12; and model 1 + MMA. Nonsignificant factors (P values > 0.10) were eliminated. To account for the complex survey design of NHANES, all statistical analyses were conducted using SAS-callable SUDAAN software, version 11 (Research Triangle Institute).
Results
Prevalence of PCP
In this cross-sectional cohort study in 2867 US adults aged ≥60 y, the prevalence of PCP, defined by DSST score, was 15.1% (95% CI: 12.9%, 17.5%). Prevalence of PCP was significantly higher among older adults; non-Hispanic blacks and Hispanics; those with less than a high school education or lower PIR; those who were not married/living together, or smoked; those without folic acid or vitamin B-12 supplement use; those with elevated serum MMA; those with high creatinine, ACR, or eGFR <60 mL · min−1 · 1.73 m−2; and those with anemia, macrocytosis, diabetes, or arthritis (Tables 1 and 2, Supplemental Table 1).
TABLE 1.
Prevalence and distribution of poor cognitive performance among adults aged ≥60 y by demographic characteristics and risk factors, NHANES 2011–20141
| Characteristics and risk factors | Sample n (weighted %) |
Distribution of cognitive performance, % ± SE | |||
|---|---|---|---|---|---|
| Prevalence (95% CI) |
Poor (n = 750) |
Not poor (n = 2117) |
OR (95% CI) |
||
| Total, n | 2867 | 15.1 ± 1.1 | 84.9 ± 1.1 | ||
| Folic acid source | |||||
| ECGPs | 1355 (40.8) | 19.1 (15.3, 23.6) | 51.8 ± 2.8 | 38.8 ± 1.7 | Ref. |
| ECGPs + RTEs | 503 (18.2) | 15.7 (12.3, 19.9) | 19.0 ± 2.3 | 18.1 ± 1.2 | 0.8 (0.5, 1.1) |
| ECGPs + SUPs | 673 (25.6) | 11.3 (8.6, 14.7) | 19.2 ± 1.7 | 26.8 ± 1.5 | 0.5 (0.4, 0.7) |
| ECGPs + RTEs + SUPs | 336 (15.4) | 9.9 (7.0, 13.7) | 10.0 ± 1.6 | 16.3 ± 1.4 | 0.5 (0.3, 0.8) |
| Folic acid supplement use,2 μg | |||||
| Nonusers | 1858 (59.0) | 18.1 (15.1, 21.5) | 70.8 ± 2.2 | 56.9 ± 1.5 | Ref. |
| Users | 1009 (41.0) | 10.8 (8.8, 13.2) | 29.2 ± 2.2 | 43.1 ± 1.5 | 0.6 (0.4, 0.7) |
| ≥10003 | 33 (1.3) | 20.6 (8.5, 42.1)4,5 | 1.7 ± 0.9 | 1.2 ± 0.3 | 1.2 (0.4, 3.4) |
| 800 to <1000 | 77 (3.6) | 6.5 (2.6, 15.2)5 | 1.5 ± 0.7 | 3.9 ± 0.5 | 0.3 (0.1, 0.9) |
| 400 to <800 | 650 (27.2) | 11.1 (9.1, 13.6) | 20.1 ± 1.7 | 28.5 ± 1.4 | 0.6 (0.4, 0.7) |
| <400 | 249 (8.9) | 9.9 (6.5, 14.8) | 5.9 ± 0.9 | 9.5 ± 1.0 | 0.5 (0.3, 0.8) |
| RBC folate concentrations, nmol/L | |||||
| ≥1496 | 974 (39.5) | 13.1 (11.0, 15.5) | 34.2 ± 2.1 | 40.4 ± 1.8 | 0.6 (0.4, 0.8) |
| 800 to <1496 | 1386 (47.1) | 15.3 (12.8, 18.2) | 47.8 ± 2.1 | 46.9 ± 1.7 | 0.7 (0.5, 1.0) |
| <800 | 507 (13.5) | 20.1 (14.9, 26.7) | 18.0 ± 2.0 | 12.7 ± 1.1 | Ref. |
| Serum folate concentrations, nmol/L | |||||
| ≥59 | 996 (40.0) | 12.2 (10.1, 14.7) | 32.8 ± 1.9 | 41.3 ± 1.1 | Ref. |
| <59 | 1809 (60.0) | 16.7 (14.1, 19.7) | 67.2 ± 1.9 | 58.7 ± 1.1 | 1.4 (1.2, 1.8) |
| Vitamin B-12 supplement use,2 μg | |||||
| Nonusers | 1747 (55.1) | 18.0 (14.8, 21.7) | 65.7 ± 2.4 | 53.3 ± 1.5 | Ref. |
| Users | 1120 (44.9) | 11.5 (9.6, 13.8) | 34.3 ± 2.3 | 46.7 ± 2.5 | 0.6 (0.5, 0.8) |
| >25 | 424 (16.7) | 10.3 (7.9, 13.3) | 11.4 ± 1.5 | 17.7 ± 1.2 | 0.5 (0.4, 0.8) |
| >6 to 25 | 417 (16.9) | 11.4 (8.6, 14.9) | 12.7 ± 1.3 | 17.6 ± 1.3 | 0.6 (0.4, 0.8) |
| ≤6 | 279 (11.3) | 13.6 (9.5, 19.1) | 10.2 ± 1.9 | 11.5 ± 0.9 | 0.7 (0.5, 1.1) |
| Vitamin B-12 insufficiency, pmol/L | |||||
| ≥258, Not insufficient | 2240 (80.3) | 14.2 (12.3, 16.3) | 77.1 ± 2.3 | 80.9 ± 1.4 | 0.8 (0.6, 1.1) |
| <258, Insufficiency | 551 (19.7) | 17.2 (12.2, 23.8) | 22.9 ± 2.3 | 19.1 ± 1.4 | Ref. |
| >150, Not deficient | 2716 (97.2) | 14.6 (12.5, 16.9) | 95.8 ± 1.3 | 97.4 ± 0.6 | 0.6 (0.3, 1.2) |
| ≤150, Deficiency | 75 (2.8) | 22.2 (11.8, 37.7) | 4.2 ± 1.3 | 2.6 ± 0.6 | Ref. |
| MMA concentrations, nmol/L | |||||
| >260, Elevated | 510 (17.8) | 25.3 (19.9, 31.6) | 30.4 ± 2.1 | 15.6 ± 1.1 | Ref. |
| ≤260, Normal | 2285 (82.2) | 12.6 (10.8, 14.6) | 69.6 ± 2.1 | 84.4 ± 1.1 | 0.4 (0.3, 0.5) |
| Creatinine, mg/dL | |||||
| ≥1.3, High | 367 (10.5) | 29.9 (22.0, 39.3) | 21.1 ± 1.9 | 8.6 ± 1.0 | 2.8 (1.9, 4.2) |
| <1.3, Not high | 2432 (89.5) | 13.0 (11.2, 15.2) | 78.9 ± 1.9 | 91.4 ± 1.0 | Ref. |
| Albumin:creatinine ratio, mg/g | |||||
| >300 | 95 (2.1) | 43.3 (31.4, 56.1) | 6.1 ± 1.3 | 1.4 ± 0.2 | 5.2 (3.1, 8.8) |
| 30–300 | 449 (13.3) | 25.6 (20.7, 31.1) | 22.5 ± 1.8 | 11.6 ± 0.7 | 2.4 (1.9, 2.9) |
| <30 | 2323 (84.6) | 12.7 (10.9, 14.8) | 71.4 ± 1.8 | 87.0 ± 0.8 | Ref. |
| Estimated glomerular filtration rate, mL · min−1 · 1.73 m−2 | |||||
| ≥60 | 2235 (78.3) | 12.3 (10.6, 14.2) | 63.8 ± 2.1 | 80.9 ± 1.0 | Ref. |
| <60 | 632 (21.7) | 25.2 (20.1, 31.1) | 36.2 ± 2.1 | 19.1 ± 1.0 | 2.4 (1.9, 3.1) |
ECGP, enriched cereal-grain product; RTE, ready-to–eat cereal; SUP, dietary supplement containing folic acid.
Participant has used dietary supplement in the past 30 d. Average daily use (μg).
Df are less than suggested, i.e., 5; imprecise as a population estimate, interpret with caution (43).
Data not suppressed as allowed due to CI >0.30; imprecise as a population estimate, interpret with caution (43).
Data not suppressed as allowed due to relative CI width >130%; imprecise as a population estimate, interpret with caution (43).
TABLE 2.
Prevalence of PCP among adults aged ≥60 y by demographic characteristics and risk factors according to RBC folate concentrations, NHANES 2011–20141
| Characteristics | Sample n, weighted % |
PCP prevalence, % |
PCP prevalence by RBC folate concentration categories, % (95% CI) | ||
|---|---|---|---|---|---|
| Low (<800 nmol/L) |
Moderate (800–1495 nmol/L) |
High (≥1496 nmol/L) |
|||
| Overall | 2867 | 15.1 (12.9, 17.5) | 20.1 (14.9, 26.7) | 15.3 (12.8, 18.2) | 13.1 (11.0, 15.5) |
| Age, y | |||||
| 60–64 | 901 (32.2) | 7.0 (5.2, 9.4) | 12.1 (8.2, 17.4) | 7.9 (5.6, 11.1) | 2.7 (1.3, 5.7)2 |
| 65–69 | 649 (23.6) | 10.4 (7.7, 14.0) | 12.3 (7.8, 19.1) | 12.3 (9.2, 16.2) | 7.2 (4.2, 12.1) |
| 70–74 | 537 (18.9) | 16.0 (12.5, 20.3) | 25.0 (13.5, 41.8) | 14.4 (10.8, 19.1) | 15.0 (11.1, 20.1) |
| 75–79 | 312 (10.7) | 23.5 (18.2, 29.6) | 44.3 (27.9, 62.0) | 27.8 (20.7, 36.2) | 16.1 (10.3, 20.3) |
| ≥80 | 468 (14.6) | 33.1 (29.1, 37.3) | 53.7 (32.7, 73.5)3 | 35.0 (28.3, 42.3) | 28.9 (23.6, 34.9) |
| Gender | |||||
| Male | 1408 (45.8) | 15.3 (12.5, 18.5) | 22.1 (16.3, 29.4) | 15.3 (12.2, 19.0) | 12.4 (9.7, 15.8) |
| Female | 1459 (54.2) | 14.9 (12.4, 17.9) | 18.1 (12.1, 26.2) | 15.4 (11.8, 19.7) | 13.5 (10.6, 17.0) |
| Race/ethnicity | |||||
| Non-Hispanic white | 1374 (79.6) | 10.4 (8.8, 12.2) | 11.9 (7.0, 19.3) | 9.7 (7.6, 12.2) | 10.7 (8.8, 13.0) |
| Non-Hispanic black | 664 (8.2) | 37.5 (32.5, 42.7) | 37.4 (28.5, 47.2) | 38.8 (31.9, 46.2) | 34.4 (26.3, 43.5) |
| All Hispanic | 546 (7.1) | 44.2 (40.0, 48.4) | 46.1 (35.0, 57.6) | 43.6 (39.2, 48.1) | 44.0 (34.3, 54.2) |
| Other | 283 (5.1) | 12.0 (8.0, 17.6) | 19.0 (11.9, 29.1) | 11.2 (6.3, 19.3) | 9.5 (5.6, 15.7) |
| Education | |||||
| Less than high school | 728 (16.0) | 44.7 (38.9, 50.6) | 44.2 (29.0, 60.6)3 | 52.6 (45.6, 59.6) | 36.1 (29.5, 43.2) |
| High school/GED | 678 (23.7) | 17.0 (13.3, 21.5) | 20.7 (13.1, 31.2) | 19.1 (13.9, 25.7) | 12.9 (9.7, 17.0) |
| More than high school | 1459 (61.5) | 6.7 (5.6, 7.9) | 9.8 (5.6, 16.8) | 5.4 (4.0, 7.2) | 7.3 (5.4, 9.8) |
| Marital status | |||||
| Married/living together | 1659 (65.0) | 11.0 (9.2, 13.1) | 16.4 (11.3, 23.2) | 11.7 (9.5, 14.3) | 8.6 (6.9, 10.8) |
| Widowed/divorced/separated | 1043 (30.7) | 23.0 (19.1, 27.5) | 26.5 (18.7, 36.1) | 21.9 (16.5, 28.4) | 23.0 (18.3, 28.4) |
| Never married | 163 (4.3) | 20.0 (12.1, 31.0) | 19.7 (9.2, 37.2)2 | 23.9 (12.6, 40.6) | 13.8 (6.2, 27.8)2 |
| PIR4 | |||||
| ≥4.0 | 698 (26.5) | 4.5 (3.0, 6.7) | 7.8 (2.8, 19.8)2 | 3.6 (2.0, 6.2) | 4.8 (2.8, 8.0) |
| 2–3.9 | 707 (26.9) | 11.0 (8.9, 13.4) | 8.2 (4.3, 15.1)2 | 11.5 (8.1, 16.1) | 11.2 (7.4, 16.6) |
| 1–1.9 | 776 (29.5) | 26.6 (21.7, 32.3) | 30.3 (20.3, 42.6) | 27.6 (21.2, 35.1) | 23.8 (18.4, 30.2) |
| <1.0 | 449 (17.1) | 41.1 (32.4, 50.3) | 42.9 (27.2, 60.2)3 | 43.7 (34.3, 53.6) | 35.7 (26.8, 45.7) |
| BMI status, kg/m2 | |||||
| ≥30 | 1063 (37.4) | 14.3 (10.9, 18.6) | 19.5 (11.3, 31.7) | 14.9 (11.2, 19.5) | 12.0 (8.6, 16.5) |
| 25 to >30 | 1005 (36.3) | 14.5 (12.2, 17.1) | 19.0 (14.1, 25.1) | 14.2 (11.1, 17.9) | 13.1 (10.0, 17.0) |
| <25 | 758 (26.2) | 15.8 (12.6, 19.6) | 20.7 (13.1, 31.2) | 16.8 (12.1, 22.8) | 12.7 (8.2, 19.1) |
| Smoking status5 | |||||
| Nonsmoker | 2363 (85.9) | 14.1 (11.8, 16.7) | 19.9 (14.2, 27.0) | 14.7 (12.1, 17.6) | 11.9 (9.6, 14.6) |
| Smoker | 452 (14.1) | 20.1 (15.1, 26.2) | 20.5 (14.5, 28.1) | 19.3 (12.7, 28.4) | 21.1 (13.4, 31.7) |
| Folic acid source | |||||
| ECGPs | 1355 (40.8) | 19.1 (15.3, 23.6) | 23.7 (16.5, 32.9) | 16.3 (13.0, 20.2) | 22.5 (15.9, 30.7) |
| ECGPs + RTEs | 503 (18.2) | 15.7 (12.3, 19.9) | 9.7 (4.7, 18.8)2 | 17.2 (12.8, 22.8) | 15.2 (9.5, 23.4) |
| ECGPs + SUPs | 673 (25.6) | 11.3 (8.6, 14.7) | 11.9 (4.6, 27.5)2 | 13.7 (9.2, 19.8) | 9.9 (7.3, 13.4) |
| ECGPs + RTEs + SUPs | 336 (15.4) | 9.9 (7.0, 13.7) | NA | 8.3 (4.5, 14.9) | 10.6 (7.2, 15.3) |
| Folic acid supplement use,6 μg | |||||
| Nonusers | 1858 (64.8) | 18.1 (15.1, 21.5) | 21.4 (15.6, 28.7) | 16.6 (13.6, 19.9) | 19.1 (15.4, 23.3) |
| ≥10007 | 33 (1.2) | 20.6 (8.5, 42.1)2, 3 | NA | 72.3 (34.3, 92.9)3 | 14.0 (5.2, 32.4)2 |
| 800 to <1000 | 77 (2.7) | 6.5 (2.6, 15.2)2 | NA | 7.2 (1.5, 28.2)2 | 6.6 (2.4, 16.9)2 |
| 400 to <800 | 650 (22.7) | 11.1 (9.1, 13.6) | 12.8 (3.3, 38.5)2,3 | 11.0 (7.9, 15.2) | 11.1 (8.4, 14.4) |
| <400 | 249 (8.7) | 9.9 (6.5, 14.8) | 10.6 (3.1, 30.9)2 | 11.9 (7.0, 19.3) | 8.2 (5.2, 12.8) |
| Serum folate, nmol/L | |||||
| ≥59 | 996 (40.0) | 12.2 (10.1, 14.7) | 21.8 (6.1, 54.4)2,3 | 13.2 (8.9, 19.1) | 11.8 (9.7, 14.2) |
| <59 | 1809 (60.0) | 16.7 (14.1, 19.7) | 19.7 (14.4, 26.4) | 15.9 (13.4, 18.7) | 15.7 (12.0, 20.2) |
| Vitamin B-12 supplement use,4 μg | |||||
| Nonusers | 1747 (55.1) | 18.0 (14.8, 21.7) | 21.9 (16.2, 28.9) | 16.4 (13.2, 20.2) | 18.6 (14.8, 23.1) |
| >25 | 424 (16.7) | 10.3 (7.9, 13.3) | 9.5 (2.9, 27.1)2 | 12.8 (8.5, 19.0) | 8.6 (6.2, 11.9) |
| >6 to 25 | 417 (16.9) | 11.4 (8.6, 14.9) | 7.8 (3.1, 18.5)2 | 12.1 (8.0, 17.9) | 11.3 (8.0, 15.7) |
| ≤6 | 279 (11.3) | 13.6 (9.5, 19.1) | 28.2 (7.9, 64.1)2,3 | 14.8 (8.4, 24.7) | 12.3 (7.6, 19.2) |
| Vitamin B-12 insufficiency, pmol/L | |||||
| >258, Not insufficient | 2240 (80.3) | 14.2 (12.3, 16.3) | 18.4 (13.6, 24.5) | 14.9 (12.6, 17.5) | 12.4 (10.2, 14.9) |
| ≤258, Insufficiency | 551 (19.7) | 17.2 (12.2, 23.8) | 23.3 (15.2, 34.0) | 15.7 (10.0, 23.7) | 14.2 (7.8, 24.6) |
| MMA concentrations, nmol/L | |||||
| >260, Elevated | 2285 (82.2) | 25.3 (19.9, 31.6) | 36.9 (23.0, 53.3)3 | 22.8 (16.4, 30.9) | 23.8 (17.1, 32.2) |
| ≤260, Normal | 510 (17.8) | 12.6 (10.8, 14.6) | 16.1 (12.0, 21.3) | 13.5 (11.0, 16.4) | 10.2 (8.5, 12.3) |
| Creatinine, mg/dL | |||||
| ≥1.3, High | 367 (10.5) | 29.9 (22.0, 39.3) | 41.0 (24.5, 59.9)3 | 31.5 (21.6, 43.6) | 26.1 (17.2, 37.5) |
| <1.3, Not high | 2432 (89.5) | 13.0 (11.2, 15.2) | 18.2 (12.9, 25.1) | 13.5 (11.1, 16.3) | 10.6 (8.4, 13.3) |
| Albumin:creatinine ratio, mg/g | |||||
| >300 | 95 (2.1) | 43.3 (31.4, 56.1) | 32.9 (11.7, 64.3)2,3 | 43.3 (28.1, 59.8)3 | 47.3 (22.3, 73.8)3 |
| 30–300 | 449 (13.3) | 25.6 (20.7, 31.1) | 30.9 (16.1, 51.1)3 | 29.5 (21.9, 38.5) | 20.7 (15.4, 27.4) |
| <30 | 2323 (84.6) | 12.7 (10.9, 14.8) | 18.4 (13.3, 24.9) | 12.7 (10.4, 15.3) | 10.7 (8.6, 13.3) |
| Estimated glomerular filtration rate, mL · min−1 · 1.73 m−2 | |||||
| ≥60 | 2235 (78.3) | 12.3 (10.6, 14.2) | 17.0 (12.0, 23.6) | 12.8 (10.7, 15.4) | 9.7 (7.7, 12.0) |
| <60 | 632 (21.7) | 25.2 (20.1, 31.1) | 35.7 (23.4, 50.2) | 26.2 (20.0, 33.7)2 | 22.2 (17.5, 27.6) |
| Anemia8 | |||||
| Yes | 486 (12.5) | 31.6 (24.7, 39.4) | 40.4 (27.2, 55.2)2 | 33.6 (24.3, 44.4)2 | 27.9 (19.6, 38.1)2 |
| No | 2379 (87.5) | 12.7 (10.8, 14.9) | 17.8 (12.7, 24.2) | 13.2 (10.8, 15.9) | 10.3 (8.2, 12.9) |
| Macrocytosis8 | |||||
| Yes | 155 (5.3) | 23.0 (15.9, 32.1) | 13.3 (5.5, 28.7) | 27.5 (15.0, 45.1)2 | 23.0 (12.4, 38.6)2 |
| No | 2710 (94.7) | 14.6 (12.5, 17.1) | 20.7 (15.1, 27.6) | 14.8 (12.3, 17.6) | 12.4 (10.4, 14.8) |
| Diabetes8 | |||||
| Yes | 665 (20.1) | 22.5 (17.0, 29.1) | 29.4 (16.4, 46.9)2, 3 | 22.0 (17.4, 27.4) | 20.8 (12.9, 31.9) |
| No | 2068 (79.9) | 13.3 (11.4, 15.5) | 18.0 (13.2, 24.1) | 13.5 (11.0, 16.5) | 11.4 (8.9, 14.4) |
| Cancer8 | |||||
| Yes | 559 (23.5) | 13.4 (10.5, 17.0) | 18.8 (11.2, 29.8) | 12.9 (9.5, 17.2) | 12.7 (9.1, 17.4) |
| No | 2305 (76.5) | 15.6 (13.1, 18.5) | 20.4 (14.7, 27.8) | 16.0 (13.0, 19.6) | 13.2 (10.7, 16.2) |
| Arthritis8 | |||||
| Yes | 1393 (49.6) | 17.6 (14.9, 20.7) | 23.4 (16.9, 31.6) | 18.8 (15.6, 22.5) | 15.0 (12.0, 18.5) |
| No | 1464 (50.4) | 12.2 (10.0, 14.7) | 17.3 (11.5, 25.3) | 11.8 (9.2, 15.0) | 10.5 (8.1, 13.7) |
| Heart disease8 | |||||
| Yes | 261 (9.5) | 16.7 (11.5, 23.7) | 23.3 (9.3, 47.3)2,3 | 15.9 (9.5, 25.4) | 15.9 (11.3, 21.9) |
| No | 2591 (90.5) | 14.7 (12.6, 17.1) | 19.6 (14.4, 26.2) | 15.1 (12.6, 17.9) | 12.6 (10.4, 15.2) |
ECGP, enriched cereal-grain product; GED, General Educational Development; PCP, poor cognitive performance; PIR, poverty:income ratio; RTE, ready-to–eat cereal; SUP, dietary supplement containing folic acid, NA, not applicable.
Data not suppressed as allowed due to relative CI width >130%; imprecise as a population estimate, interpret with caution (43).
Data not suppressed as allowed due to CI >0.30; imprecise as a population estimate, interpret with caution (43).
PIR is defined as the ratio of self-reported family income to the federal poverty threshold, accounting for family size, year, and state; higher values correspond to higher socioeconomic status.
Nonsmoker: serum cotinine concentrations ≤10 ng/mL; smoker: serum cotinine concentrations >10 ng/mL.
Average daily use of dietary supplement (μg).
Df are less than suggested, i.e., 5; imprecise as a population estimate, interpret with caution (43).
Self-report; participants were asked if they had ever been told by a doctor or other health professional that they had the health condition of interest.
Cognitive performance by folic acid intake and health status
In bivariate analyses, consuming multiple sources of folic acid was associated with significantly lower odds of PCP (Table 1). Compared with consuming only ECGPs, persons consuming ECGPs + SUPs or ECGPs + RTEs + SUPs had lower odds of PCP (OR: 0.5; 95% CI: 0.4, 0.7 and OR: 0.5; 95% CI: 0.3, 0.8, respectively). Furthermore, people with higher (as opposed to low, <30 mg/g) ACRs had significantly increased odds of PCP (OR: 2.4; 95% CI: 1.9, 2.9 among those with ACR of 30–300 mg/g and OR: 5.2; 95% CI: 3.1, 8.8 among those with ACR >300 mg/g) (Table 1).
Compared with persons with usual folic acid intake <400 μg/d, persons with intake ≥400 μg/d had significantly reduced odds of PCP (OR: 0.50; 95% CI: 0.37, 0.66) (Table 3). The finding remained significant after adjustment. UMFA was not associated with cognitive performance and therefore removed from the multivariable models.
TABLE 3.
ORs of poor cognitive performance in adults aged ≥60 y by usual folic acid intake and blood folate concentrations (categoric variables), NHANES 2011–20141
| Cutoff | n | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 1 + serum vitamin B-12 |
Model 1 + MMA | ||||
| Usual folic acid intake | ||||||
| NTDs2 | ≥400 μg | 840 | 0.50 (0.37, 0.66) | 0.62 (0.44, 0.90) | 0.66 (0.46, 0.96) | 0.66 (0.46, 0.96) |
| UL | ≥1000 μg | 21 | 0.50 (0.10, 2.43) | 0.64 (0.11, 3.75) | 0.81 (0.14, 4.66) | 0.83 (0.15, 4.70) |
| Serum folate percentile | ||||||
| 25th | ≥30 nmol/L | 2120 | 0.83 (0.63, 1.10) | 0.84 (0.57, 1.22) | 0.88 (0.59, 1.30) | 0.88 (0.58, 1.33) |
| 50th | ≥46 nmol/L | 1404 | 0.71 (0.55, 0.91) | 0.65 (0.47, 0.91) | 0.69 (0.50, 0.97) | 0.69 (0.49, 0.97) |
| Lit3 | ≥59 nmol/L | 996 | 0.69 (0.57, 0.85) | 0.59 (0.42, 0.82) | 0.63 (0.45, 0.87) | 0.63 (0.45, 0.88) |
| 75th | ≥71 nmol/L | 692 | 0.78 (0.61, 0.98) | 0.62 (0.42, 0.92) | 0.65 (0.43, 0.99) | 0.66 (0.44, 0.99) |
| 90th | ≥97 nmol/L | 265 | 1.03 (0.74, 1.44) | 0.67 (0.42, 1.05) | 0.72 (0.45, 1.17) | 0.73 (0.45, 1.18) |
| 95th | ≥114 nmol/L | 128 | 1.14 (0.72, 1.79) | 0.58 (0.28, 1.21) | 0.63 (0.30, 1.31) | 0.62 (0.30, 1.27) |
| 97th | ≥167 nmol/L | 26 | 1.13 (0.42, 3.00) | 0.73 (0.23, 2.35) | 0.81 (0.25, 2.63) | 0.78 (0.27, 2.30) |
| RBC folate percentile | ||||||
| 25th | ≥897 nmol/L | 2152 | 0.62 (0.49, 0.79) | 0.67 (0.46, 0.99) | 0.71 (0.51, 1.00) | 0.71 (0.49, 1.03) |
| 50th | ≥1220 nmol/L | 1460 | 0.73 (0.57, 0.93) | 0.74 (0.52, 1.04) | 0.75 (0.52, 1.06) | 0.75 (0.52, 1.07) |
| Lit3 | ≥1609 nmol/L | 810 | 0.81 (0.65, 1.00) | 0.69 (0.50, 0.97) | 0.75 (0.53, 1.05) | 0.71 (0.51, 1.00) |
| 75th | ≥1670 nmol/L | 734 | 0.86 (0.69, 1.08) | 0.71 (0.52, 0.98) | 0.77 (0.56, 1.06) | 0.74 (0.53, 1.02) |
| 90th | ≥2160 nmol/L | 286 | 0.99 (0.72, 1.36) | 0.64 (0.43, 0.95) | 0.67 (0.44, 1.02) | 0.65 (0.45, 0.95) |
| Lit3 | ≥2397 nmol/L | 205 | 0.89 (0.57, 1.39) | 0.63 (0.37, 1.06) | 0.67 (0.39, 1.15) | 0.64 (0.40, 1.03) |
| 95th | ≥2620 nmol/L | 145 | 1.13 (0.69, 1.85) | 0.82 (0.47, 1.43) | 0.85 (0.48, 1.53) | 0.80 (0.48, 1.34) |
| 97th | ≥3000 nmol/L | 77 | 2.03 (1.06, 3.87) | 1.41 (0.72, 2.77) | 1.47 (0.72, 3.02) | 1.34 (0.69, 2.62) |
Model 1 adjusted for age, gender, ethnicity, marital status, education, smoking, poverty:income ratio, estimated glomerular filtration rate, and albumin:creatinine ratio. Reference for ≥400 and ≥1000 is <400 and <1000, respectively; reference for ≥30, ≥46, ≥59, ≥71, ≥97, ≥114, and ≥167 is <30, <46, <59, <71, <97, <114, and <167, respectively; and reference for ≥897, ≥1220, ≥1609, ≥1670, ≥2160, ≥2397, ≥2620, and ≥3000 is <897, <1220, <1609, <1670, <2160, <2397, <2620, and <3000, respectively. Lit, literature; MMA, methylmalonic acid; NTD, neural tube defect; UL, tolerable upper intake level.
The recommended daily intake amount to prevent NTDs is ≥400 μg.
Commonly used values in the literature.
Nutritional risk factors for PCP
PCP was more common among persons who had lower RBC folate concentrations (<800 nmol/L, prevalence: 20.1%; 95% CI: 14.9%, 26.7%) than among those with higher RBC folate concentrations (800 to <1496 nmol/L, prevalence: 15.3%; 95% CI: 12.8%, 18.2% and ≥1496 nmol/L, prevalence: 13.1%; 95% CI: 11.0%, 15.5%). Similarly, PCP was more common among persons who had lower serum folate concentrations (Tables 1 and 2). Supplement use was associated with lower unadjusted odds of PCP among those reporting vitamin B-12 use (OR: 0.6; 95% CI: 0.5, 0.8) or folic acid use (OR: 0.6; 95% CI: 0.4, 0.7). Among supplement users, PCP was more common among persons who had lower vitamin B-12 supplement use (≤6 μg, prevalence: 13.6%; 95% CI: 9.5%, 19.1%) than among those with higher vitamin B-12 supplement use (>6 to 25 μg, prevalence: 11.4%; 95% CI: 8.6%, 14.9% and >25 μg, prevalence: 10.3%; 95% CI: 7.9%, 13.3%) (Tables 1 and 2).
Compared with persons without PCP, those with PCP were less likely to use supplements containing folic acid or vitamin B-12 (mean ± SEE: 34.4% ± 2.4% and 47.5% ± 1.6%, respectively) (data not shown).
Among vitamin B-12–insufficient adults, 18.0% ± 1.6% (mean ± SEE) reported taking a vitamin B-12 supplement. Among participants with high folate and insufficient vitamin B-12 concentrations, 34.3% ± 11.5% (mean ± SEE) reported taking vitamin B-12–containing supplements. Concurrent high folate and insufficient vitamin B-12 concentrations were not associated with PCP.
Cognitive performance (as measured by DSST) by serum folate concentrations
High serum folate concentration (≥90th percentile) was not significantly associated with PCP, before and after adjustment (Table 3). Persons with high folate and low vitamin B-12 did not have significantly higher odds of PCP than those with high folate and normal vitamin B-12 (OR: 1.46; 95% CI: 0.78, 2.71) (data not shown). In addition, persons with serum folate in the 50th–75th percentiles (≥46 to ≥71 nmol/L) had significantly decreased odds of PCP compared with persons with serum folate <46 to <71 nmol/L in all models (Table 3).
Cognitive performance (as measured by DSST) by RBC folate concentrations
As a continuous measure, increasing RBC folate concentration did not significantly increase the odds of PCP after adjustment for important covariates across all measured percentile groups. At the 25th, 75th, and 90th percentiles, high RBC folate was protective of PCP (Table 3). Persons with RBC folate in the 90th percentile had decreased odds of PCP after adjustment for important covariates (aOR: 0.64; 95% CI: 0.43, 0.95) and after adjustment for important covariates and MMA (aOR: 0.65; 95% CI: 0.45, 0.95). Persons with RBC folate in the 97th percentile (≥3000 nmol/L) had increased odds of PCP compared with those with RBC folate <3000 nmol/L, but the association became nonsignificant after adjustment in all models. The mean ±SE intake of folic acid (478.46 ± 26.88 μg) among persons with RBC folate concentrations ≥3000 nmol/L was less than half the UL (≥1000 μg/d) (data not shown).
Cognitive performance as measured by other (CERAD and AF) tests
Results from the sensitivity analysis showed that high folate concentrations were not associated with decreased cognitive performance (Supplemental Table 2).
Interaction of folate, vitamin B-12, and MMA
Compared with persons with normal folate and normal vitamin B-12 concentrations, persons with high folate and normal vitamin B-12 concentrations had significantly lower odds of PCP (aOR: 0.61; 95% CI: 0.45, 0.83), and persons with high folate and insufficient vitamin B-12 concentrations had no significant difference in PCP (aOR: 0.89; 95% CI: 0.36, 2.22) (Table 4). Increasing MMA concentrations were associated with increased odds of PCP (aOR: 1.57; 95% CI: 1.19, 2.06), whereas Decreasing vitamin B-12 concentrations were not (aOR: 0.93; 95% CI: 0.64, 1.36) (Table 4). Adjusting for eGFR alone significantly attenuated the risk associated with high folate and high MMA (unadjusted OR: 1.81; 95% CI: 1.23, 2.66; adjusted eGFR only OR: 1.31; 95% CI: 0.88, 1.94; fully adjusted model aOR: 1.23; 95% CI: 0.63, 2.40) (Table 4).
TABLE 4.
ORs of poor cognitive performance in adults aged ≥60 y by RBC and serum folate, serum vitamin B-12, and serum MMA concentrations (categoric + continuous variables), NHANES 2011–20141
| Folate and vitamin B-12 status | n | Unadjusted | Adjusted model |
|---|---|---|---|
| NF and N B-12 | 342 | Ref. | Ref. |
| NF and I B-12 | 125 | 1.07 (0.70, 1.63) | 1.11 (0.65, 1.90) |
| HF and N B-12 | 218* | 0.69 (0.55, 0.88)* | 0.61 (0.45, 0.83)* |
| HF and I B-12 | 37 | 1.01 (0.60, 1.71) | 0.89 (0.36, 2.22) |
| NF and N MMA | 353 | Ref. | Ref. |
| NF and H MMA2 | 116* | 2.10 (1.40, 3.17)* | 1.48 (0.92, 2.37) |
| HF and N MMA | 191* | 0.66 (0.52, 0.84)* | 0.56 (0.40, 0.78)* |
| HF and H MMA3 | 64* | 1.81 (1.23, 2.66)* | 1.23 (0.63, 2.40) |
| Model 14 | |||
| RBC folate only | 2867* | 0.71 (0.56, 0.91)* | 0.66 (0.45, 0.96)* |
| Serum folate only | 2805* | 0.77 (0.64, 0.92)* | 0.65 (0.48, 0.89)* |
| Serum vitamin B-12 only | 2791 | 0.93 (0.67, 1.29) | 0.93 (0.64, 1.36) |
| MMA only | 2795* | 2.09 (1.66, 2.64)* | 1.57 (1.19, 2.06)* |
| Model 1 ± RBC folate and serum vitamin B-125 | |||
| RBC folate | 2791 | 0.68 (0.51, 0.91) | 0.65 (0.42, 1.00) |
| Serum vitamin B-12 | 2791 | 1.02 (0.72, 1.47) | 1.05 (0.69, 1.60) |
| Model 1 ± serum folate and vitamin B-125 | |||
| Serum folate | 2771* | 0.77 (0.63, 0.93)* | 0.65 (0.46, 0.90)* |
| Serum vitamin B-12 | 2771 | 1.02 (0.72, 1.43) | 1.06 (0.71, 1.57) |
| Model 1 ± RBC folate and MMA5 | |||
| RBC folate | 2795* | 0.71 (0.56, 0.90)* | 0.69 (0.48, 1.00) |
| MMA | 2795* | 2.08 (1.64, 2.63)* | 1.52 (1.13, 2.03)* |
| Model 1 ± serum folate and MMA5 | |||
| Serum folate | 2775* | 0.78 (0.65, 0.94)* | 0.67 (0.49, 0.93)* |
| MMA | 2775* | 2.04 (1.59, 2.61)* | 1.43 (1.03, 1.98)* |
Values are ORs (95% CIs) unless otherwise indicated.
Values are significant. HF defined as serum folate ≥59 nmol/L or RBC folate ≥1609 nmol/L; NF defined as serum folate <59 nmol/L or RBC folate <1609 nmol/L; I B-12 as vitamin B-12 ≤258 pmol/L; N B-12 as vitamin B-12 >258 pmol/L; H MMA as MMA >260 nmol/L; N MMA as MMA ≤260 nmol/L). ACR, albumin:creatinine ratio; eGFR, estimated glomerular filtration rate; HF, high folate; H MMA, elevated MMA; I B-12, insufficient vitamin B-12; MMA, methylmalonic acid; N B-12, normal vitamin B-12; NF, normal folate; N MMA, normal MMA; PIR, poverty:income ratio.
Adjusted for only eGFR OR: 1.73 (95% CI: 1.13, 2.65).
Adjusted for only eGFR OR: 1.31 (95% CI: 0.88, 1.94).
Model 1 adjusted for age, gender, ethnicity, marital status, education, smoking, PIR, eGFR, and ACR.
Continuous models adjusted for age, gender, ethnicity, marital status, education, smoking, PIR, eGFR, and ACR.
Discussion
In this large, population-based representative sample of adults aged ≥60 y, we were unable to detect an association of PCP with concurrent high folate and insufficient vitamin B-12 concentrations. Also, in the presence of normal vitamin B-12 concentrations, high folate concentrations were associated with better cognitive performance. Those with usual intake of folic acid ≥400 μg had better cognitive performance than those with intake <400 μg; those with intake at or exceeding the UL did not have significant differences in cognitive performance compared with those with intake below the UL. Previous studies reported that high serum folate was associated with higher risk of poorer cognitive performance (18, 19, 24). However, in our study high serum folate concentrations were not associated with low scores on cognitive tests even after adjusting for vitamin B-12 or MMA. In fact, from the 50th to the 75th percentile, high serum folate was significantly associated with lower odds of PCP. Our results were consistent between 3 different (categoric and continuous) measures of folate status: folic acid intake, as well as serum and RBC folate concentrations. We did find suggestions that high folate and low vitamin B-12 may warrant clinical evaluation and appropriate medical intervention, specifically for vitamin B-12 malabsorption and/or kidney disease.
Cutoffs for folate and vitamin B-12 values vary in the literature, with many combining markers in different combinations (18, 19, 24). Our analysis spanned both continuous and categoric exposure variables from the 5th to the 95th percentile and highlights the lack of evidence implicating high folate in worsened cognitive performance even at the highest folates and/or among those who were vitamin B-12 insufficient. It is of critical importance to fully understand the role of high folate and low vitamin B-12. Evidence suggests that this phenomenon is an artifact of a sick population and is a reflection of vitamin B-12 malabsorption and/or conditions such as kidney failure. Although people with high folate and low vitamin B-12 are at risk of poor health outcomes, this may not be due to their high folate status or folate intake at all but an underlying health condition.
Vitamin B-12 and MMA
Among adults aged ≥60 y in the United States, the RDA of vitamin B-12 (2.4 μg) is often exceeded through diet or supplement use (17, 44). Although vitamin B-12 is widely available through SUPs or through foods containing vitamin B-12, some people may be unable to achieve adequate concentrations of vitamin B-12 owing to several factors, including diet (e.g., not eating animal products), digestive disorders, or underlying diseases (i.e., in pernicious anemia) (44, 45). Vitamin B-12 is required for utilization of any folate by the S-adenosylmethionine/S-adenosylhomocysteine (SAM/SAH) and MMA pathways (46). Vitamin B-12 deficiency can lead to an accumulation of MMA in the urine and plasma, neurologic deterioration, and is critical to diagnose (47). Difficulties with kidney excretion and vitamin B-12 malabsorption could also contribute to higher MMA concentrations and vitamin B-12 insufficiency (47). MMA is strongly tied to renal function. Previous analyses have combined vitamin B-12 and MMA status but we did not do this to allow for differential effects because assessing MMA in an independent analysis provides an alternative way of understanding insufficient vitamin B-12. MMA and vitamin B-12 concentrations do not directly correlate with each other across the measured range. We found weaker but consistent associations of PCP with vitamin B-12 concentrations than with MMA concentrations.
To raise vitamin B-12 concentrations, vitamin B-12 supplements are often recommended, and among those who do not respond adequately to this treatment, intramuscular injections are recommended by Wolffenbuttel et al. (48). To our knowledge, our study is the first to assess vitamin B-12 supplement use among those with vitamin B-12 insufficiency in the context of folate and cognitive health.
Underlying medical conditions
Among participants who had insufficient vitamin B-12 concentrations, 18% used a vitamin B-12–containing supplement. The insufficient vitamin B-12 status among these individuals despite their intake of vitamin B-12 supplements may indicate that they are unable to adequately absorb vitamin B-12. Laboratory tests that can detect and diagnose vitamin B-12 and folate deficiencies are available, relatively inexpensive, and may be warranted to quickly identify and treat individuals with malabsorption issues (29, 49). Our study supports the need for clinicians to consider routinely assessing vitamin B-12 concentrations in older adults and to consider vitamin B-12 injections as an important route if inadequate vitamin B-12 status is identified despite consumption of oral vitamin B-12 supplements.
We also examined the concern that a phenomenon occurs with “too much folic acid,” in the presence of low vitamin B-12 concentrations (18, 19, 24). We found that both high concentrations of RBC folate (a measure of long-term folate intake) and serum folate (a measure of more recent folate intake) were not associated with PCP. We further restricted our sample to those with the highest RBC folate concentrations and found that those individuals had similar folic acid intake to those with lower RBC folate concentrations. Among participants with high folate and insufficient vitamin B-12 concentrations, 34% reported taking vitamin B-12–containing supplements. This is critical for the appropriate interpretation of findings from studies of the possible impact of high folate and insufficient vitamin B-12 concentrations because these are likely confounded by malabsorption and some of these studies may need to be reinterpreted.
A small percentage of adults in our sample accumulated high RBC folate concentrations in the absence of high folic acid intake and/or folic acid supplements, which might suggest that those with the highest folate concentrations have problems with excretion. The mean intake of folic acid (478.46 ± 26.88 μg/d) among persons with RBC concentrations ≥3000 nmol/L was much lower than the folic acid UL of ≥1000 μg/d. It has been estimated that the usual median intakes of folic acid in US adults aged ≥60 y for ECGPs only, ECGPs + RTEs, ECGPs + SUPs, and ECGPs + RTEs + SUPs are 104, 238, 490, and 672 μg/d, respectively (50). These findings are suggestive that the high RBC folate concentrations are due to something other than high intake of folic acid. As such, folate accumulation may be associated with metabolic and excretion issues whereby older adults have decreased uptake by the liver or kidney or greater release from cells (47, 49). Although high folate and high MMA did have increased odds of PCP unadjusted, the affect was attenuated in a multivariate analysis including adjustments for kidney function; this is consistent with the association of high MMA concentrations with poor kidney function (51). When interpreting findings associated with high folate and insufficient vitamin B-12 concentrations, it is critical to consider comorbidities as causal for bioaccumulation and not assume high intake.
Our findings do not support the hypothesis that high folate concentrations are associated with PCP as was suggested in previous studies in older adults with high folate and low vitamin B-12 concentrations, including a recent one that combined MMA and vitamin B-12 deficiency but did not adjust for eGFR (18, 19, 24). It may be unlikely that high folate concentrations are responsible for the PCP noticed in older adults with low vitamin B-12 concentrations. Adjusting for renal function is essential and future analyses should include this variable to better understand the association. In addition to routinely assessing vitamin B-12 concentrations, it would be helpful if clinicians were aware that high folate concentrations may indicate underlying health conditions and an appropriate blood marker may need to be identified to assess issues of excretion.
Cognitive tests
Although the DSST is a sensitive and good measure of PCP, it is not comprehensive of all domains of cognitive function and primarily measures processing speed, attention span, and working memory (19, 24, 27). Our results do not support a need to limit folic acid intake among older adults under common dosages (e.g., 400–800 μg/d), although large dosages (e.g., ≥4000 μg/d) of any substance are generally unnecessary. The literature indicates that folate-containing supplements may help improve long-term cognitive function in older adults who are healthy and have normal vitamin B-12 concentrations (18, 19). Both folate and vitamin B-12 play an important role in cognitive health.
Strengths and limitations
This study had several strengths and limitations. The use of the NHANES for this study is a strength because it provides a nationally representative data set that includes rich and comprehensive information on dietary, demographic, and lifestyle factors; objective cognitive performance testing; as well as biological samples to account for known key confounders. Other strengths of our study include the use of multiple definitions and cutoffs to assess folic acid (supplement and food) intake, vitamin B-12 supplement intake, and folate and vitamin B-12 biomarker concentrations; the use of two 24-h dietary recalls using an established statistical method to estimate usual daily intakes of folic acid; and adjustment for potential confounders. Although self-reported information provided by respondents could be imprecise, our study was strengthened by the availability of biomarker data (52). One limitation of the study is that actual folic acid in foods may be higher or lower than that estimated in the nutrient database (53). In addition, we could not assess baseline responses and examine whether PCP demonstrates poorer cognition over time or if a respondent typically answers poorly on these types of tests. However, residual confounding remains a possibility and as a cross-sectional study, we cannot determine temporal causality.
Implications and impact
Our results provide useful insight into dietary patterns in adults ≥60 y old as well as potential benefits of proper supplementation and routine clinical testing for vitamin B-12. Identifying older adults who are more likely to be vitamin B-12 insufficient is essential to prevent potential cognitive issues regardless of folate status. Clinicians might consider alternative routes for vitamin B-12 delivery, such as offering vitamin B-12 injections to overcome malabsorption issues in affected individuals. Careful considerations and adjustments are needed in analyses of high folate concentrations because these may be indicative of underlying health conditions and not high intake.
Conclusion
The idea that “high” folic acid intake impairs cognition persists despite evidence to the contrary. Insufficient vitamin B-12 concentrations have been consistently associated with PCP. Policy makers are encouraged to consider multipronged approaches, including fortification to address vitamin B-12 insufficiency and active clinical management to assess absorption issues. Early detection and treatment of vitamin B-12 deficiency or excretion issues are essential to prevent cognitive and hematologic issues.
Supplementary Material
Acknowledgments
We thank Arick Wang for his assistance calculating the usual intake using the National Cancer Institute method.
Abbreviations used:
- ACR
albumin:creatinine ratio
- AF
Animal Fluency
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- DSST
Digit Symbol Substitution Test
- ECGP
enriched cereal-grain product
- eGFR
estimated glomerular filtration rate
- LC–MS/MS
liquid chromatography–tandem mass spectrometry
- MEC
mobile examination center
- MMA
methylmalonic acid
- NHANES
National Health and Nutrition Examination Survey
- NTD
neural tube defect
- PCP
poor cognitive performance
- PIR
poverty:income ratio
- RBC
red blood cell
- RTE
ready-to–eat cereal
- SAM/SAH
adenosylmethionine/S-adenosylhomocysteine
- SUP
dietary supplement
- UL
tolerable upper intake level
- UMFA
unmetabolized folic acid
- US
United States
- WAIS III
Wechsler Adult Intelligence Scale, Third Edition
Footnotes
The authors’ responsibilities were as follows—KSC: initiated the project; KSC and LFY: designed the research and had primary responsibility for the final content; MES, CER, and YPQ: conducted the research and analyzed the data; MES: wrote the initial draft of the paper; KSC, LFY, CER, YPQ, and CAT: contributed to revisions; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
The authors reported no funding received for this study.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data Availability
Publicly available data sets were analyzed in this study. The data can be found here: https://wwwn.cdc.gov/nchs/nhanes/default.aspx (accessed 20 August, 2019).
References
- 1.NIH National Institute on Aging (NIA). Cognitive health and older adults: reduce risks to cognitive health[Internet]. Bethesda (MD): NIH NIA; 2020. [Accessed January 2020]. Available from: https://www.nia.nih.gov/health/cognitive-health-and-older-adults#reduce%20risks. [Google Scholar]
- 2.Kremen WS, Panizzon MS, Cannon TD. Genetics and neuropsychology: a merger whose time has come. Neuropsychology 2016;30(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul KC, Haan M, Mayeda ER, Ritz BR. Ambient air pollution, noise, and late-life cognitive decline and dementia risk. Annu Rev Public Health 2019;40:203–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaffe K. Modifiable risk factors and prevention of dementia: what is the latest evidence? JAMA Intern Med 2018;178(2):281–2. [DOI] [PubMed] [Google Scholar]
- 5.Anderson LA, McConnell SR. Cognitive health: an emerging public health issue. Alzheimers Dement 2007;3(2S, 2 Suppl):S70–3. [DOI] [PubMed] [Google Scholar]
- 6.Brody DJ, Kramarow EA, Taylor CA, McGuire LC. Cognitive performance in adults aged 60 and over: National Health and Nutrition Examination Survey, 2011–2014. Natl Health Stat Report 2019;(126):1–23. [PubMed] [Google Scholar]
- 7.Kotagal V, Langa KM, Plassman BL, Fisher GG, Giordani BJ, Wallace RB, Burke JR, Steffens DC, Kabeto M, Albin RL, Foster NL. Factors associated with cognitive evaluations in the United States. Neurology 2015;84(1):64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med 2008;148(6):427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 2001;56(1):37–42. [DOI] [PubMed] [Google Scholar]
- 10.Gestuvo M, Hung W. Common dietary supplements for cognitive health. Aging Health 2012;8(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stover PJ. Vitamin B12 and older adults. Curr Opin Clin Nutr Metab Care 2010;13(1):24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace TC, Frankenfeld CL, Frei B, Shah AV, Yu C-R, van Klinken BJ-W, Adeleke M. Multivitamin/multimineral supplement use is associated with increased micronutrient intakes and biomarkers and decreased prevalence of inadequacies and deficiencies in middle-aged and older adults in the United States. J Nutr Gerontol Geriatr 2019;38(4):307–28. [DOI] [PubMed] [Google Scholar]
- 13.Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, Picciano MF, McDowell M, Sempos C. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994). NCHS Data Brief 2011;(61):1–8. [PubMed] [Google Scholar]
- 14.Gahche JJ, Bailey RL, Potischman N, Dwyer JT. Dietary supplement use was very high among older adults in the United States in 2011–2014. J Nutr 2017;147(10):1968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson MA. If high folic acid aggravates vitamin B12 deficiency what should be done about it? Nutr Rev 2007;65(10):451–8. [DOI] [PubMed] [Google Scholar]
- 16.Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr 2009;89(2):693S–6S. [DOI] [PubMed] [Google Scholar]
- 17.Mishra S, Stierman B, Gahche JJ, Potischman N. Dietary supplement use among adults: United States, 2017–2018. NCHS Data Brief 2021;(399):1–8. [PubMed] [Google Scholar]
- 18.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr 2010;91(6):1733–44. [DOI] [PubMed] [Google Scholar]
- 19.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr 2007;85(1):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry RJ. Lack of historical evidence to support folic acid exacerbation of the neuropathy caused by vitamin B12 deficiency. Am J Clin Nutr 2019;110(3):554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FDA. Food Standards: amendment of standards of identity for enriched grain products to require addition of folic acid; final rule. Fed Regist 1996;61(44):8781–97. [Google Scholar]
- 22.Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, Frohnert B, Kirby RS. Updated estimates of neural tube defects prevented by mandatory folic acid fortification – United States, 1995–2011. MMWR Morb Mortal Wkly Rep 2015;64(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Odewole OA, Williamson RS, Zakai NA, Berry RJ, Judd SE, Qi YP, Adedinsewo DA, Oakley, Jr GP. Near-elimination of folate-deficiency anemia by mandatory folic acid fortification in older US adults: Reasons for Geographic and Racial Differences in Stroke study 2003–2007. Am J Clin Nutr 2013;98(4):1042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey RL, Jun S, Murphy L, Green R, Gahche JJ, Dwyer JT, Potischman N, McCabe GP, Miller JW. High folic acid or folate combined with low vitamin B-12 status: potential but inconsistent association with cognitive function in a nationally representative cross-sectional sample of US older adults participating in the NHANES. Am J Clin Nutr 2020;112(6):1547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruvada P, Stover PJ, Mason JB, Bailey RL, Davis CD, Field MS, Finnell RH, Garza C, Green R, Gueant J-L, et al. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am J Clin Nutr 2020;112(5):1390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics (NCHS). About the National Health and Nutrition Examination Survey. [Internet]. Hyattsville (MD): NCHS; 2017. [Accessed August 2019]. Available from: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. [Google Scholar]
- 27.Wechsler D. Wechsler Adult Intelligence Scale—Third Edition. Administration and scoring manual. San Antonio (TX): The Psychological Corporation; 1997. [Google Scholar]
- 28.Chen SP, Bhattacharya J, Pershing S. Association of vision loss with cognition in older adults. JAMA Ophthalmol 2017;135(9):963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989;39(9):1159–65. [DOI] [PubMed] [Google Scholar]
- 30.Sotaniemi M, Pulliainen V, Hokkanen L, Pirttilä T, Hallikainen I, Soininen H, Hänninen T. CERAD-neuropsychological battery in screening mild Alzheimer’s disease. Acta Neurol Scand 2012;125(1):16–23. [DOI] [PubMed] [Google Scholar]
- 31.Tooze JA, Midthune D, Dodd KW, Freedman LS, Krebs-Smith SM, Subar AF, Guenther PM, Carroll RJ, Kipnis V. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J Am Diet Assoc 2006;106(10):1575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser 2013;13(23):1–45. [PMC free article] [PubMed] [Google Scholar]
- 33.Oberlin BS, Tangney CC, Gustashaw KA, Rasmussen HE. Vitamin B12 deficiency in relation to functional disabilities. Nutrients 2013;5(11):4462–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey: 2011–2012 data documentation, codebook, and frequencies: vitamin B12 (VITB12_G). [Internet]. Hyattsville (MD): NCHS; 2014. [Accessed August 2019]. Available from: https://wwwn.cdc.gov/nchs/nhanes/2011-2012/VITB12_G.htm. [Google Scholar]
- 35.Aparicio-Ugarriza R, Palacios G, Alder M, González-Gross M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin Chem Lab Med 2015;53(8):1149–59. [DOI] [PubMed] [Google Scholar]
- 36.National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey: 2011–2012 data documentation, codebook, and frequencies: methylmalonic acid (MMA_G). [Internet]. Hyattsville (MD): NCHS; 2018. [Accessed August 2019]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/MMA_G.htm. [Google Scholar]
- 37.Yetley EA, Pfeiffer CM, Phinney KW, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, Randy Curtin L, Durazo-Arvizu RA, et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr 2011;94(1):313S–21S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klee GG. Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B12 and folate. Clin Chem 2000;46(8):1277–83. [PubMed] [Google Scholar]
- 39.National Center for Health Statistics (NCHS). Laboratory procedure manual: total folate. [Internet]. Hyattsville (MD): NCHS; 2014. [Accessed August 2019]. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/folate_g_met_rbc.pdf. [Google Scholar]
- 40.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfeiffer CM, Sternberg MR, Fazili Z, Yetley EA, Lacher DA, Bailey RL, Johnson CL. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J Nutr 2015;145(3):520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fazili Z, Sternberg MR, Potischman N, Wang CY, Storandt RJ, Yeung L, Yamini S, Gahche JJ, Juan W, Qi YP, et al. Demographic, physiologic, and lifestyle characteristics observed with serum total folate differ among folate forms: cross-sectional data from fasting samples in the NHANES 2011–2016. J Nutr 2020;150(4):851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker JD, Talih M, Malec DJ, Beresovsky V, Carroll M, Gonzalez JF Jr. National Center for Health Statistics data presentation standards for proportions. Vital Health Stat 2017;2(175):1–22. Available at: https://www.cdc.gov/nchs/data/series/sr_02/sr02_175.pdf. [PubMed] [Google Scholar]
- 44.Institute of Medicine. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): The National Academies Press; 1998. [PubMed] [Google Scholar]
- 45.Subar AF, Krebs-Smith SM, Cook A, Kahle LL. Dietary sources of nutrients among US adults, 1989 to 1991. J Am Diet Assoc 1998;98(5):537–47. [DOI] [PubMed] [Google Scholar]
- 46.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr 2012;3(1):21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Supakul S, Chabrun F, Genebrier S, N’Guyen M, Valarche G, Derieppe A, Villoteau A, Lacombe V, Urbanski G. Diagnostic performances of urinary methylmalonic acid/creatinine ratio in vitamin B12 deficiency. J Clin Med 2020;9(8):2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolffenbuttel BHR, Wouters HJCM, Heiner-Fokkema MR, van der Klauw MM. The many faces of cobalamin (vitamin B12) deficiency. Mayo Clin Proc Innov Qual Outcomes 2019;3(2):200–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shipton MJ, Thachil J. Vitamin B12 deficiency – a 21st century perspective. Clin Med 2015;15(2):145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Q, Cogswell ME, Hamner HC, Carriquiry A, Bailey LB, Pfeiffer CM, Berry RJ. Folic acid source, usual intake, and folate and vitamin B-12 status in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2006. Am J Clin Nutr 2010;91(1):64–72. [DOI] [PubMed] [Google Scholar]
- 51.Riphagen IJ, Minović I, Groothof D, Post A, Eggersdorfer ML, Kootstra-Ros JE, de Borst MH, Navis G, Muskiet FAJ, Kema IP, et al. Methylmalonic acid, vitamin B12, renal function, and risk of all-cause mortality in the general population: results from the prospective Lifelines-MINUTHE study. BMC Med 2020;18(1):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bailey RL, Fulgoni VL, Taylor CL, Pfeiffer CM, Thuppal SV, McCabe GP, Yetley EA, Correspondence of folate dietary intake and biomarker data. Am J Clin Nutr 2017;105(6):1336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr 2008;88(2):324–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available data sets were analyzed in this study. The data can be found here: https://wwwn.cdc.gov/nchs/nhanes/default.aspx (accessed 20 August, 2019).