Abstract
Background.
The systolic and diastolic dyssynchrony is physiologically related, but measure different left ventricular mechanisms. Left ventricular systolic mechanical dyssynchrony (systolic LVMD) has shown significant clinical values in improving cardiac resynchronization therapy (CRT) response in the heart failure patients with dilated cardiomyopathy (DCM). Our recent study demonstrated that LV diastolic dyssynchrony (diastolic LVMD) parameters have important prognostic values for DCM patients. However, there are a limited number of studies about the clinical value of diastolic LVMD for CRT. This study aims to explore the predictive values of both systolic LVMD and diastolic LVMD for CRT in DCM patients.
Methods.
Eighty-four consecutive CRT patients with both DCM and complete left bundle branch block (CLBBB) who received gated resting SPECT MPI at baseline were included in the present study. The phase analysis technique was applied on resting gated short-axis SPECT MPI images to measure systolic LVMD and diastolic LVMD, characterized by phase standard deviation (PSD) and phase histogram bandwidth (PBW). CRT response was defined as ≥ 5% improvement of LVEF at 6-month follow-up. Variables with P < 0.10 in the univariate analysis were included in the multivariate cox analysis.
Results.
During the follow-up period, 59.5% (50 of 84) patients were CRT responders. The univariate cox regression analysis showed that at baseline QRS duration, non-sustained ventricular tachycardia (NS-VT), systolic PSD, systolic PBW, diastolic PSD, diastolic PBW, scar burden and LV lead in the scarred myocardium were statistically significantly associated with CRT response. The multivariate cox regression analysis showed that QRS duration, NS-VT, systolic PSD, systolic PBW, diastolic PSD, and diastolic PBW were independent predictive factors for CRT response. Furthermore, the rate of CRT response was 94.4% (17 of 18) in patients whose LV lead was in the segments with both the first three late contraction and the first three late relaxation; by contrast, the rate of CRT response was only 6.7% (1 of 15, P < 0.000) in patients whose LV lead was in the segments with neither the first three late contraction nor the first three late relaxation.
Conclusion.
Both systolic LVMD and diastolic LVMD from gated SPECT MPI have important predictive values for CRT response in DCM patients. Pacing at LV segments with both late contraction and late relaxation has potential to increase the CRT response.
Keywords: Dilated cardiomyopathy, Diastolic dyssynchrony, Single photon emission computed tomography, Phase analysis, Cardiac resynchronization therapy
INTRODUCTION
Cardiac resynchronization therapy (CRT) has been proved to be an effective treatment option for patients with chronic heart failure, left ventricular ejection fraction (LVEF) <35% and enlarged QRS.1,2 However, 30% to 40% of CRT patients show non-response to CRT without improved clinical symptoms and cardiac function.3 It has been demonstrated that CRT non-response is related to left ventricular (LV) myocardial viability, mechanical dyssynchrony, and lead position.4 Left ventricular systolic dyssynchrony (systolic LVMD) is an independent predictor of CRT response in heart failure patients.5 More importantly, LV lead should be placed away from the scar and at or near the latest site of contraction onset.6 Phase analysis on gated SPECT MPI is a well-established technique to measure LVMD and it has been successfully used to predict acute change in LV synchrony and patient outcome after CRT.7
Most of the existing studies mainly focused on systolic dyssynchrony and the timing of onset of mechanical contraction. Recent research has shown that CRT is related with worsening or new occurrence of systolic and/or diastolic dyssynchrony by tissue Doppler echocardiography.8,9 Using echocardiography, Verbrugge et al found that an improved diastolic filling time was associated with favorable reverse LV remodeling and improved clinical outcomes in CRT patients.8 Above studies implied a potential importance of diastolic dyssynchrony in selecting CRT candidates. Gated SPECT has good correlation with tissue Doppler imaging and speckle tracking echocardiography for the assessment of diastolic LVMD.9,10 Moreover, compared to other methods such as echocardiography, phase analysis on gated SPECT MPI to measure mechanical dyssynchrony is well-standardized, largely automatic and highly reproducible.4 However, there are limited studies about the predictive value of diastolic LVMD for CRT by SPECT MPI.11,12 This study aims to explore the predictive values of both systolic LVMD and diastolic LVMD from gated SPECT MPI in CRT patients with dilated cardiomyopathy.
METHODS
Patient Population
Eighty-four CRT patients with both DCM and complete left bundle branch block (CLBBB) who received gated resting SPECT MPI were consecutively enrolled in the present study at the First Affiliated Nanjing Medical University Hospital from September 2009 to August 2018. DCM was diagnosed by checking patient’s history and an exclusion of other etiological factors that might cause LV dysfunction combined with a low LVEF (≤ 45%) by echocardiography according to the recent criteria.13 The indications of CRT in this study included (1) sinus rhythm; (2) LV systolic dysfunction (LVEF ≤ 35%); (3) typical LBBB morphology; (4) New York Heart Association (NYHA) functional class from II to IV; and (5) optimal medical therapy for at least 3 months before CRT implantation. Patients with atrial fibrillation, right bundle branch block, and those being upgraded from right ventricular pacing were excluded. Coronary angiography or dual-source computed tomography was performed in all individuals, and none of them had epicardial coronary artery stenosis greater than 50%. Functional capacity of the patients was assessed according to the NYHA functional classification. The study was approved by the Institutional Ethical Committee of the First Affiliated Hospital of Nanjing Medical University, and informed consent was obtained from all patients.
Electrocardiography
A 12-lead surface electrocardiogram (ECG) was recorded during hospitalization. The QRS duration was measured according to the widest QRS complex among the 12 leads of ECG. Premature ventricular contractions (PVC) were recorded in 24-hour Holter monitoring. Nonsustained ventricular tachycardia (NS-VT) was confirmed by runs of beats arising from the ventricles lasting for at least three beats and persisting less than 30 seconds with cycle length of less than 600 milliseconds in a 24-hour Holter monitoring.
Echocardiography
LV function was assessed twice by two experienced ultrasound experts and the mean value was recorded. All the ultrasound experts were blinded to the other clinical data before and 6 months after CRT implantation.
LV volumes and LVEF were measured by the 2-dimensional modified biplane Simpson method.
Gated Myocardial Perfusion SPECT
The resting ECG-gated SPECT MPI scan was performed approximately 60 minutes after injection of 20 to 30 mCi of Tc-99m sestamibi before CRT implantation. The MPI images were acquired on a dual-headed camera (CardioMD, Philips Medical Systems) with a standard protocol. The imaging parameters included a 20% energy window around 140 KeV, 180° orbit, 32 steps with 25 seconds per step, 8-bin gating and 64 planar projections per gate. Image reconstruction and reorientation were performed with Emory Reconstruction Toolbox (ERToolbox; Syntermed, Atlanta, GA). All the SPECT planar images were reconstructed by ordered subset expectation maximization (OSEM) with 3 iterations and 10 subsets, and then filtered by a Butterworth low-pass filter with a cutoff frequency of 0.3 cycles/cm and an order of 10 subsets.
To identify the LV contour parameters, resulting gated short-axis SPECT MPI images were input into an interactive tool. These parameters and image data were then submitted to an automatic myocardial sampling algorithm which searched in 3D for maximal count circumferential profiles in each cardiac frame. Subsequently, the samples were used by a multi-harmonic phase analysis tool to assess systolic LVMD and diastolic LVMD based on the Fourier approximation. The 1-harmonic and 3-harmonic Fourier approximations were employed to calculate the onset of mechanical contraction and relaxation for each sample, respectively.14 As illustrated in Figure 1, the phase polar maps were generated to visualize the systolic and diastolic dyssynchrony and identify the late contracting and relaxing segments. Phase standard deviation (PSD) and 95% width of phase histogram bandwidth (PBW) were used to measure the global LV mechanical dyssynchrony.
Figure 1.
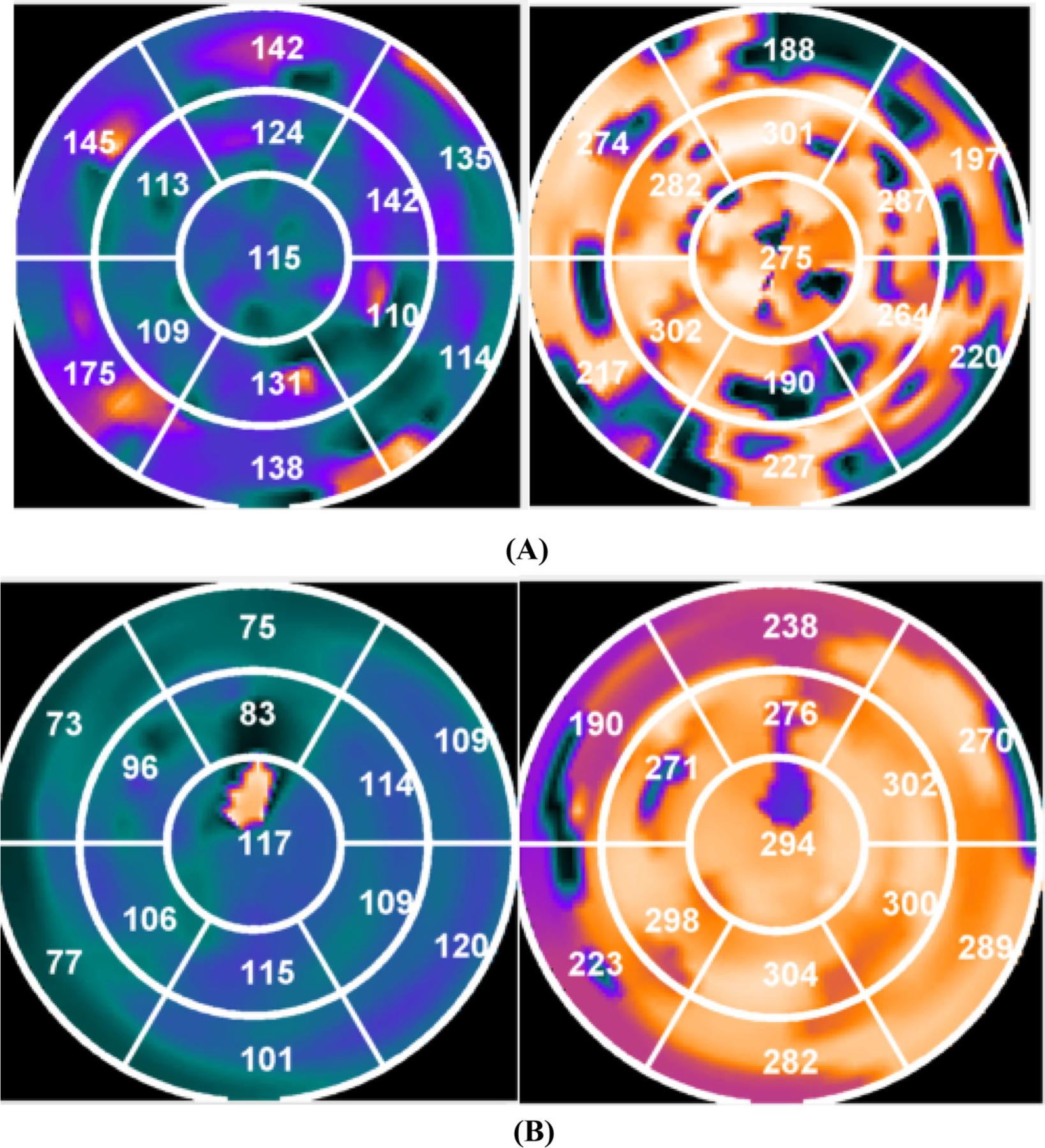
Polar maps showing systolic and diastolic dyssynchrony. (A) shows a patient who didn’t respond to CRT. The latest three contracting segments were in the septal and anterior wall and the latest three relaxing segments were in the septal, anterior, and anterolateral wall. The left ventricular lead was placed in the posterolateral wall. (B) shows a patient who responded to CRT. Both the latest three contracting and latest three relaxing segments were all in the posterolateral wall. The left ventricular lead was placed in the posterolateral wall.
CRT Implantation
All patients underwent implantation of a CRT or CRT-D. The devices were implanted according to standard procedures. The right ventricular (RV) lead was systematically implanted at the RV apex or septum, and the position of the LV lead was steadily implanted in one branch vein by experienced electrophysiologists targeting the anterolateral, lateral, or posterolateral coronary veins. The final position was decided by good stability in coronary veins, satisfactory pacing threshold and no phrenic nerve capture. The LV lead position was confirmed by fluoroscopic venograms in the right anterior oblique RAO 30° and left anterior oblique (LAO) 45° after implantation. LV concordance was defined as the concordance of LV lead position at the first three late contracting segments and the first three late relaxing segments after excluding segment with more than 50% scar.
Follow Up After CRT Implantation
The follow-up was performed by phone contact with patients or family members, as well as checking hospital and government records of death. The primary endpoint of this study was CRT response. The secondary endpoint was all-cause mortality. CRT response was defined as ≥ 5% improvement of LVEF at 6-month follow-up. Patients were divided into two groups according to the follow-up data (CRT response group vs CRT non-response group).
Statistical Analysis
Statistical analysis was performed with SPSS version 21.0 (SPSS Inc, Chicago, IL). Categorical variables were expressed as numbers or percentages, and group comparisons were performed by the Chi-square test or Fisher test. Comparisons between two groups were performed using student t-tests for normally distributed continuous variables. The degree of mechanical dyssynchrony was stratified according to the Cutoff values which was defined as 2 SDs of the PBW and PSD mechanical dyssynchrony. The contributions of the 2 dyssynchrony measures (PBW and PSD) were examined using logistic proportional hazards regression modeling for the CRT response. A Wilcoxon comparison test was used for nonnormally distributed continuous variables and a Chi-square test was used for categorical variables. After the tests were run, univariate and multivariate binary logistic regression analyses were performed to evaluate odds ratios. 95% confidence intervals were used to explore the independent predictors of CRT response. Variables with P < 0.10 in the univariate analysis were included in the multivariate cox analysis. A series of multivariable models were examined to assess mechanical dyssynchrony characterized by both PBW and PSD, after adjustment for clinical variables include QRS duration, NT-proBNP, NS-VT, scar burden and LV lead in the scarred myocardium. The model for systolic LVMD refers to inclusion of both PBW and PSD (2 degrees of freedom), and similarly for diastolic LVMD. Sequential models were compared by a likelihood-ratio test. The Kaplan-Meier curve analysis was performed to compare the all-cause mortality among three patient groups according to the match between the LV lead position and latest contracting or relaxing segments. The Kaplan-Meier curve analysis was performed to compare the all-cause mortality between the CRT response group and CRT non-response group. P < 0.05 was considered to be statistically significant.
RESULTS
Baseline Characteristics
Table 1 lists the baseline characteristics of the 84 consecutive patients included in this study (63 male, 59.3 ± 11.7 years old). 32 (38.1%) patients were classified as having NYHA functional class II, 44 (52.4%) as NYHA class III, and 8 (9.5%) as NYHA class IV. All patients were treated with guideline-directed medical therapy before CRT for at least 3 months. There was no statistical difference in the use of diuretics (95.2% of patients), aldosterone(95.2% of patients), angiotensin-converting enzyme inhibitors (ACEI) or angiotensin (AT) II antagonists (81.0% of patients), beta-blockers (97.6% of patients) and digoxin (28.6% of patients) between the CRT response group and non-response group. No significant differences were noted in age, gender, hypertension, diabetes, NYHA functional class or NT-proBNP between two groups (P > 0.05). Meanwhile, baseline LV parameters including EDV, ESV, and LVEF had no significant differences (P > 0.05) between two groups. However, compared with the CRT response group, there were more NS-VT, PVCs, scar burden and wide QRS duration in the non-response group (P < 0.05). Importantly, the CRT non-response group showed more LV lead located in the scar segment (P = 0.011). Meanwhile, systolic PSD, systolic PBW, diastolic PSD, and diastolic PBW in the CRT non-response group were statistically significantly greater (P < 0.05). Table 2 shows the correlations between systolic LVMD and diastolic LVMD parameters. The correlation coefficients between systolic LVMD and diastolic LVMD parameters were 0.895 and 0.920 for PSD and PBW, respectively. The systolic LVMD parameters were significantly different from the diastolic LVMD parameters by the paired t-test (PSD: 43.9 ± 21.9 vs 54.2±23.4, P < 0.001; PBW: 156.2 ± 87.5 vs 183.2 ± 90.3, P < 0.001).
Table 1.
Baseline characteristics and LV parameters of the enrolled patients
| Variables | All(n = 84) | Non-responders (n = 34) | Responders (n = 50) | P value |
|---|---|---|---|---|
| Age(year) | 59.3±11.7 | 58.3±10.5 | 60.0±12.5 | 0.800 |
| Male (n, %) | 63 (75.0%) | 26 (76.5%) | 37 (74.0%) | 0.735 |
| Hypertension | 16 (30.8%) | 14 (41.1%) | 16 (32.0%) | 0.005 |
| Diabetes | 10 (19.2%) | 7 (21.0%) | 8 (23.5%) | 0.284 |
| QRS duration (ms) | 154.8±24.0 | 145.3±25.7 | 161.3±20.6 | 0.002 |
| NT-proBNP | 4587.7±3477.6 | 3034.7±3971.4 | 1794.4±1877.9 | 0.068 |
| NS-VT | 52 (61.9%) | 27 (79.4%) | 25 (25.0%) | 0.007 |
| PVC | 1839.4±3472.1 | 2864.5±4667.7 | 1139.6±2124.4 | 0.024 |
| NYHA classII/III/IV | 32/44/8 | 8/21/3 | 24/23/5 | 0.064 |
| Medication | ||||
| ACE inhibitors/ARBs | 68 (81.0%) | 24 (70.6%) | 44 (88.0%) | 0.204 |
| β-Blockers | 82 (97.6%) | 34 (100%) | 48 (96.0%) | 0.148 |
| Diuretics | 80 (95.2%) | 33 (97.1%) | 47 (94.0%) | 0.148 |
| Aldosterone | 80 (95.2%) | 33 (97.1%) | 47 (94.0%) | 0.148 |
| Digoxin | 24 (28.6%) | 14 (41.2%) | 10 (20.0%) | 0.036 |
| LV parameters | ||||
| EDV | 308.4±141.7 | 288.9±130.3 | 321.1±148.8 | 0.309 |
| ESV | 256.6±129.0 | 240.4±113.9 | 267.7±138.4 | 0.346 |
| LVEF (%) | 19.7±9.3 | 21.5±10.0 | 18.6±8.7 | 0.157 |
| Scar burden | 26.6±12.1 | 30.0±11.7 | 24.2±11.9 | 0.031 |
| LV lead in the scarred myocardium | 20 | 13 | 7 | 0.011 |
| Systolic PSD (o) | 43.9±21.9 | 51.3±22.2 | 38.9±20.4 | 0.010 |
| Systolic PBW (o) | 156.2±87.5 | 188.1±88.6 | 134.4±80.5 | 0.005 |
| Diastolic PSD (o) | 54.2±23.4 | 63.0±22.4 | 48.2±22.4 | 0.001 |
| Diastolic PBW (o) | 183.2±90.3 | 220.6±88.5 | 157.8±83.1 | 0.001 |
Data are expressed as mean ± SD or number (percentage)
NT-proBNP, N-terminal pro-natriuretic brain natriuretic peptide; NS-VT, non-sustained ventricular tachycardia; PVC, premature ventricular contraction; CLBBB, complete left bundle branch block; NYHA, New York Heart Association; ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blocker; EDV, end-diastolic volume; ESV, end-systolic volume; LVEF, left ventricular ejection fraction
Table 2.
Correlations between left ventricular systolic dyssynchrony and diastolic dyssynchrony
| Systolic dyssynchrony |
Diastolic dyssynchrony |
|||
|---|---|---|---|---|
| PSD (o) | PBW (o) | PSD (o) | PBW (o) | |
| Range | 10.8–102.5 | 39.0–337.0 | 7.3–109.2 | 20.0–343.0 |
| Median | 41.9 | 141.0 | 55.7 | 186.5 |
| P | 0.000 | 0.000 | ||
| R | 0.895 | 0.920 | ||
PSD, phase standard deviation; PBW, phase bandwidth; PE, phase entropy; P value by paired t-test between the systolic and diastolic parameters; R, correlation coefficient
Prediction of CRT Response
The cutoff for the degree of mechanical dyssynchrony (two SDs above mean) was: systolic PSD = 43.7°, diastolic PSD = 46.9°, systolic PBW =174.9°, and diastolic PBW = 180.5°. Most of LV leads (82 cases, 97.6%) were placed in anterolateral, lateral, posterior or posterolateral coronary veins as determined by coronary venous anatomic characteristics. In total, 50 patients were identified as CRT responders. In Table 3, the univariate cox regression analysis showed that QRS duration, non-sustained ventricular tachycardia (NS-VT), scar burden, LV lead in the scarred myocardium, systolic PSD, systolic PBW, diastolic PSD, and diastolic PBW were statistically significantly associated with CRT response. When systolic LVMD and diastolic LVMD dyssynchrony parameters (systolic PSD, systolic PBW, diastolic PSD, and diastolic PBW) were input into the multivariate cox regression models separately, those parameters were independent predictors of CRT response along with QRS duration, NT-proBNP and NS-VT (Tables 4, 5, 6, 7). Figure 2 shows an additive approach to comparing variable contributions to overall models by the global likelihood-ratio Chi-square statistics. After multivariable adjustment for clinical characteristics and electrical dyssynchrony, sequential models indicated that the addition of systolic dyssynchrony (global Chi-square statistic of 22.9 vs. 27.3; P = 0.036) or diastolic dyssynchrony (global Chi-square statistic of 22.9 vs. 28.1; P = 0.012) was more strongly associated with CRT response. However, the addition of diastolic dyssynchrony to a model of systolic dyssynchrony plus clinical variables did not provide a significant improvement in association with CRT response compared with a model without diastolic dyssynchrony (global Chi-square statistic of 27.3 vs. 28.1; P = 0.355).
Table 3.
The univariate logistic regression model for response to CRT
| Variables | P value | Hazard ratio | 95% CI |
|---|---|---|---|
| Men | 0.797 | 1.142 | 0.414–3.146 |
| Age | 0.501 | 1.0113 | 0.976–1.052 |
| Hypertension | 0.390 | 0.672 | 0.272–1.662 |
| NT-proBNP | 0.091 | 1.000 | 1.000–1.000 |
| QRS duration (ms) | 0.004 | 1.033 | 1.010–1.056 |
| NS-VT | 0.008 | 0.259 | 0.095–0.704 |
| LVEF | 0.161 | 0.967 | 0.922–1.014 |
| NYHA functional class | 0.100 | 0.549 | 0.268–1.122 |
| Scar burden | 0.036 | 0.960 | 0.924–0.997 |
| LV lead in the scarred myocardium | 0.013 | 0.263 | 0.091–0.757 |
| Systolic PSD (o) | 0.013 | 0.973 | 0.952–0.994 |
| Systolic PBW (o) | 0.007 | 0.993 | 0.987–0.998 |
| Diastolic PSD (o) | 0.006 | 0.971 | 0.950–0.992 |
| Diastolic PBW (o) | 0.003 | 0.992 | 0.986–0.997 |
CI, confidence intervals; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-natriuretic brain natriuretic peptide; NS-VT, non-sustained ventricular tachycardia
Table 4.
The multivariate Cox regression model for CRT response when including systolic phase standard deviation
| Variables | Wald | P value | Hazard ratio | 95% CI |
|---|---|---|---|---|
| Systolic SD | 4.089 | 0.043 | 0.971 | 0.944–0.999 |
| QRS duration | 6.694 | 0.010 | 1.032 | 1.008–1.058 |
| NT-proBNP | 3.663 | 0.056 | 1.000 | 1.000–1.000 |
| NS-VT | 4.316 | 0.038 | 0.298 | 0.094–0.934 |
| LV lead in the scarred myocardium | 1.620 | 0.203 | 0.432 | 0.119–1.573 |
| Scar burden | 0.079 | 0.778 | 1.007 | 0.957–1.061 |
Table 5.
The multivariate Cox regression model for CRT response when including systolic phase histogram bandwidth
| Variables | Wald | P value | Hazard ratio | 95% CI |
|---|---|---|---|---|
| Systolic BW | 4.299 | 0.038 | 0.992 | 0.985–1.000 |
| QRS duration | 7.107 | 0.008 | 1.033 | 1.0009–1.059 |
| NT-proBNP | 3.585 | 0.058 | 1.000 | 1.000–1.000 |
| NS-VT | 3.726 | 0.054 | 0.324 | 0.103–1.017 |
| LV lead in the scarred myocardium | 1.528 | 0.216 | 0.448 | 0.125–1.601 |
| Scar burden | 0.101 | 0.750 | 1.009 | 0.957–1.063 |
Table 6.
The multivariate Cox regression model for CRT response when including diastolic phase standard deviation
| Variables | Wald | P value | Hazard ratio | 95% CI |
|---|---|---|---|---|
| Diastolic SD | 4.624 | 0.032 | 0.971 | 0.945–0.997 |
| QRS duration | 6.022 | 0.014 | 1.031 | 1.006–1.056 |
| NT-proBNP | 3.534 | 0.060 | 1.000 | 1.000–1.000 |
| NS-VT | 4.652 | 0.031 | 0.281 | 0.089–0.891 |
| LV lead in the scarred myocardium | 1.167 | 0.280 | 0.489 | 0.134–1.789 |
| Scar burden | 0.003 | 0.954 | 1.001 | 0.954–1.051 |
Table 7.
The multivariate Cox regression model for CRT response when including diastolic phase histogram bandwidth
| Variables | Wald | P value | Hazard ratio | 95% CI |
|---|---|---|---|---|
| Diastolic BW | 5.114 | 0.024 | 0.992 | 0.985–0.999 |
| QRS duration | 6.625 | 0.010 | 1.032 | 1.008–1.058 |
| NT-proBNP | 3.380 | 0.066 | 1.000 | 1.000–1.000 |
| NS-VT | 4.041 | 0.044 | 0.306 | 0.096–0.971 |
| LV lead in the scarred myocardium | 1.035 | 0.309 | 0.515 | 0.143–1.851 |
| Scar burden | 0.009 | 0.924 | 1.002 | 0.955–1.053 |
Figure 2.
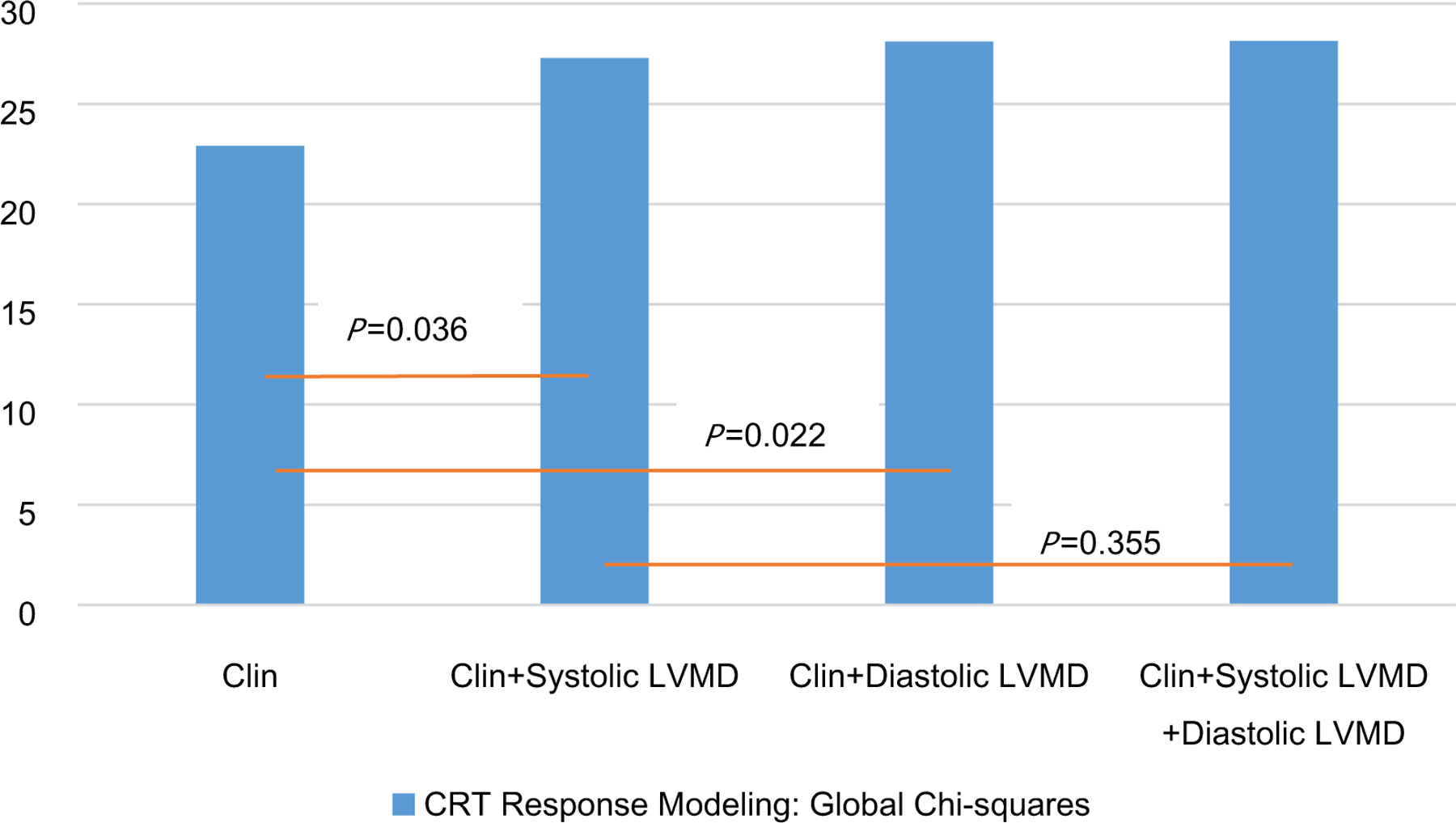
Incremental association between clinical variables (Clin), left ventricular mechanical dyssynchrony (PBW and PSD), and CRT response as indicated by the global Chi-square statistics in serial multivariable adjusted models. Clinical variables include QRS duration, NT-proBNP, NS-VT, Scar burden and LV lead in the scarred myocardium.
Furthermore, the rate of CRT response was 94.4% (17 of 18) in patients whose LV lead was in the segments of both the first three late contraction and the first three late relaxation; by contrast, the rate of CRT response was only 6.7% (1 of 15, P < 0.000) in patients whose LV lead was in the segments with neither the first three late contraction nor the first three late relaxation (Figure 3).
Figure 3.
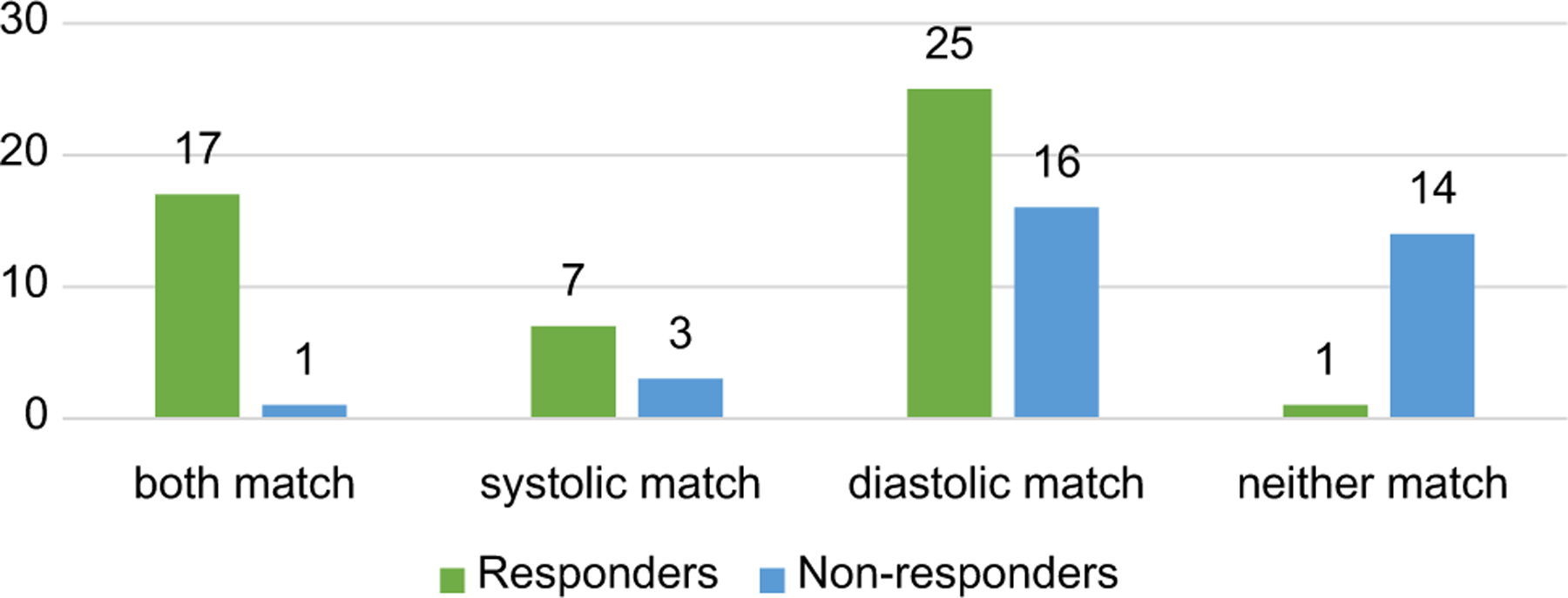
The response numbers when left ventricular lead was at the site with a different match incorporating left ventricular systolic and diastolic dyssynchrony. The green bars stand for responders and blue bars stand for non-responders. In the first category, CRT response in patients whose LV lead positions were in both the latest three contracting and latest three relaxing segments (n = 18), and only 1 case had no response. In the fourth category, CRT response in patients whose LV lead positions were neither in the latest three contracting nor latest three relaxing segments (n = 15), and totally 14 cases had no response (P < 0.000).
Follow Up
Over a mean follow-up of 35.7 months (IQR 12 to 94 months), 9 (10.6%) patients died from any cause in the entire cohort, including 1 patient (1.2%) in the CRT response group, 8 (9.4%) patients in the CRT non-response group (P = 0.001). At the patients’ 6-month follow-up, there was an overall significant increase of LVEF compared with the baseline (19.7% ± 9.3% vs. 28.3% ± 7.6%, P < 0.001); LVEF increased greater in the CRT response group compared with in the CRT non-response group (30.3% ± 12.8% vs. 5.73% ± 10.9%, P < 0.001). In terms of all-cause mortality, the CRT response group showed better long-term prognosis than the CRT non-response group (log-rank χ2 = 9.266, P = 0.002) (Figure 4). According to the match between the LV lead position and latest contracting or relaxing segments in Figure 5, there were no significant differences among the three patient groups but the result showed a trend toward different clinical outcomes (log-rank χ2 = 3.766, P = 0.152) (Figure 5).
Figure 4.
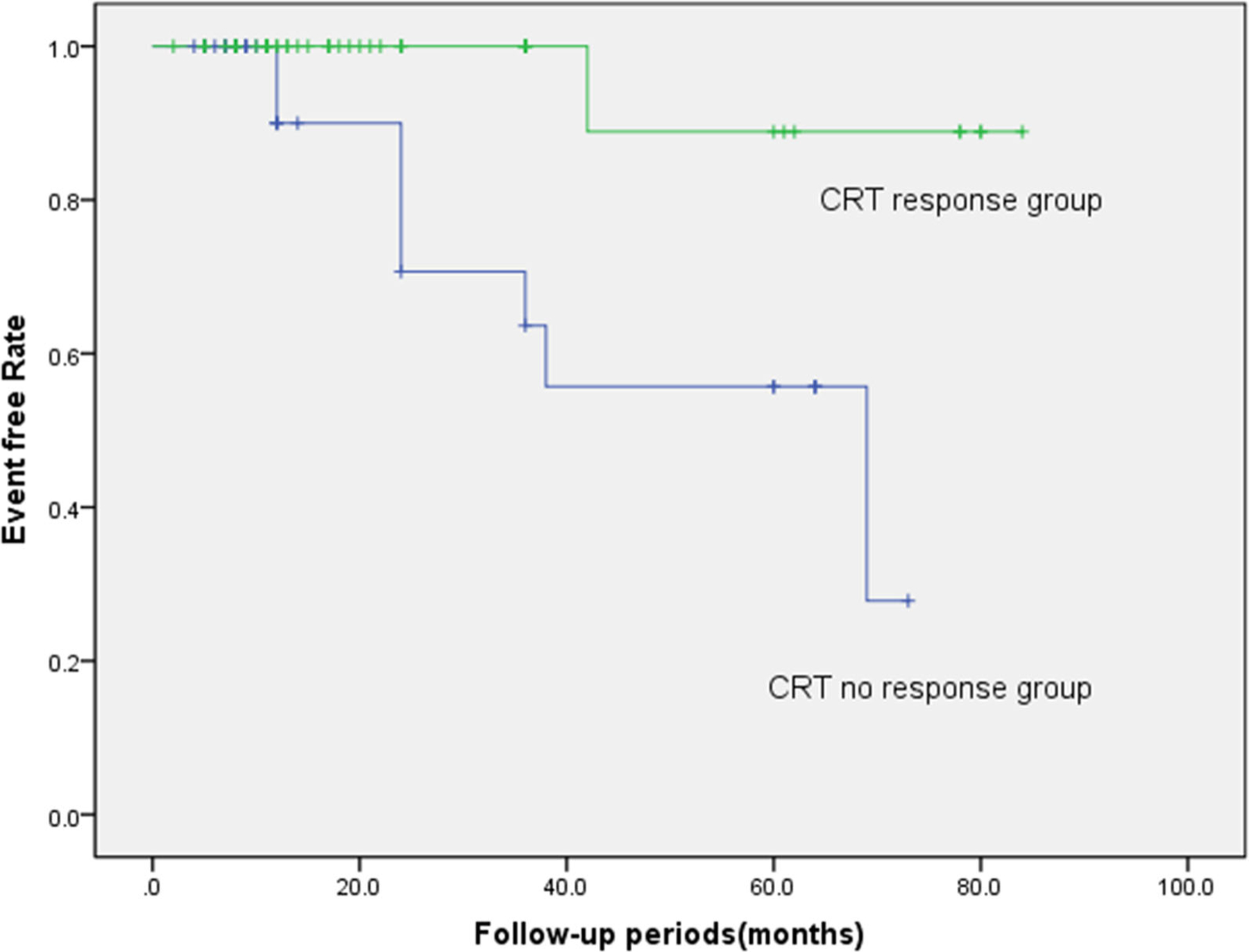
The Kaplan-Meier event-free survival curve (log-rank χ2 = 9.266, P = 0.002).
Figure 5.
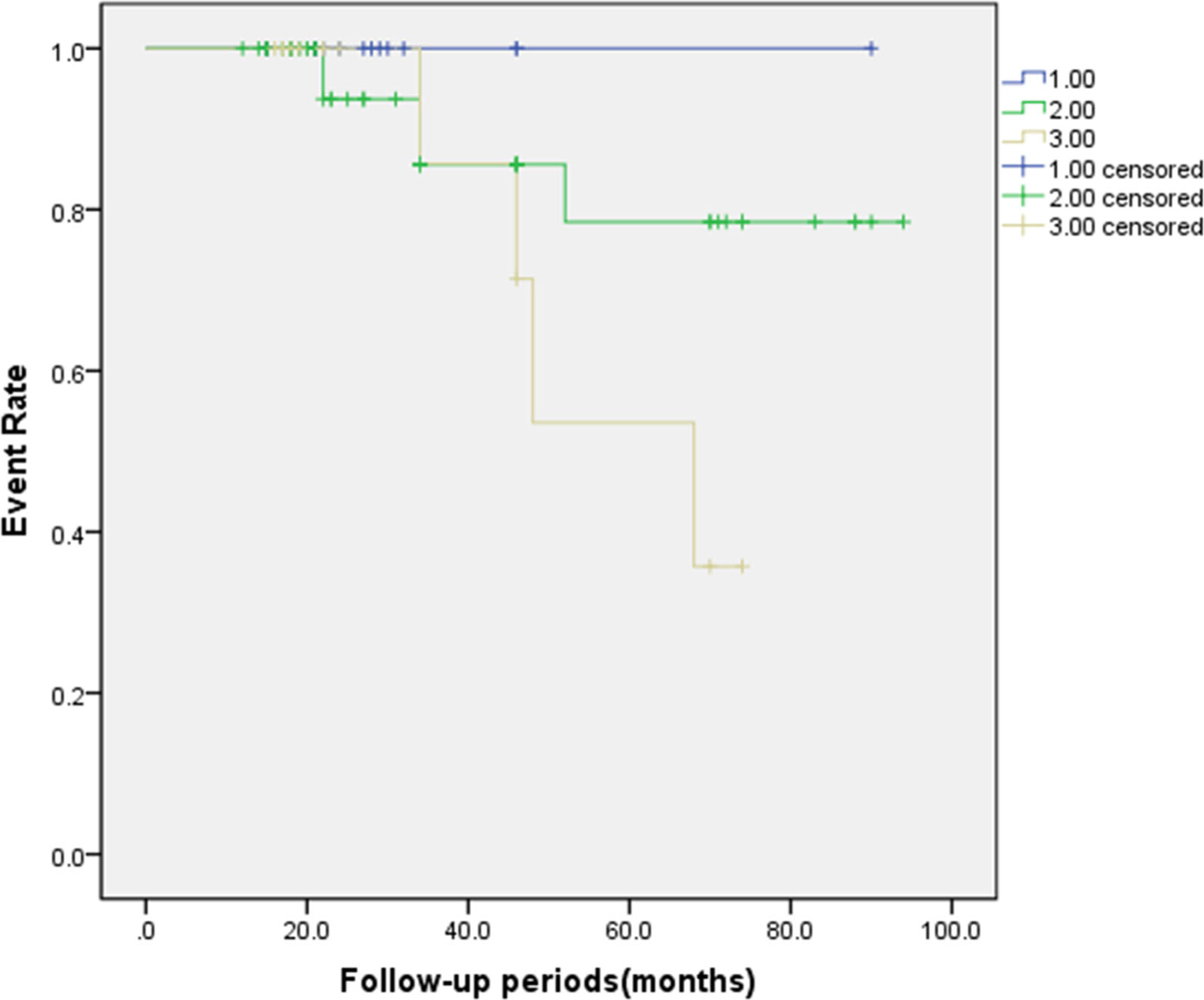
The Kaplan-Meier event-free survival curve comparing three patient groups according to the match between the LV lead position and latest contracting or relaxing segments. (log-rank χ2 = 3.766, P = 0.152). Number 1 stands for the CRT patients whose LV lead positions were in both the latest three contracting and latest three relaxing segments (both match). Number 2 stands for the CRT patients whose LV lead positions were in either the latest three contracting or latest three relaxing segments (1 match). Number 3 stands for the CRT patients whose LV lead positions were neither in the latest three contracting nor latest three relaxing segments (neither match).
DISCUSSION
The major findings of this study are: (1) both systolic LVMD and diastolic LVMD from gated SPECT MPI have important predictive values for CRT response in DCM patients; (2) Pacing at LV segments with both the late contraction and late relaxation has the potential to increase the CRT response.
Reasons of CRT Non-response
The benefits of CRT to heart failure patients have been extensively demonstrated in a large number of clinical trials. However, 30 to 40% of the patients having CRT showed non-response to CRT. Thus, it is imperative to improve HF patients’ prognosis and reducing healthcare costs to enhance CRT response. Previous studies have shown that CRT non-response might be related to LV myocardial scar.15,16 In addition, studies have shown that patients with ischemic cardiac myocarditis (ICM) have less improvement in LVEF than patients with DCM because ischemia attributes to the greater burden of scarred myocardium.17 Adelstein et al18 reported that a high scar burden in CRT patients was associated with reduced clinical outcomes and a lack of LVEF improvement. However, Riedlbauchova et al found that the extent of LV scarring did not predict the response to CRT or mortality.19 In their study, the scar burdens in both CRT response and CRT non-response groups were higher than 40%, which appears to be much higher than that reported in other studies.20,21 Our study showed that there was more higher scar burdens in CRT non-response groups. However, the scar burden was not an independent predictor for CRT response. Our explanation for this nonsignificant difference is that the myocardial scar burdens were too high in these two groups, which weakens the predictive value of the myocardial scar burden. Moreover, for CRT response it is important that the LV lead is placed away from scar 22 and at or near the site of the viable segment with the latest activation.6,23 Our study also showed that implanting LV lead in the scarred region brought negative impact on CRT response(only 7 of 20 patients responded to CRT, P = 0.011).
The presence of a prolonged QRS duration is also a strong predictor of response to CRT,24 which was also confirmed by our results. When any systolic LVMD or diastolic LVMD parameters were input into the multivariate cox regression separately, QRS duration was always an independent predictor of CRT response. NS-VT was also an independent predictor of CRT response in DCM patients in our study. It is well recognized that DCM combined with frequent ventricular ectopics would reduce the frequency of biventricular pacing and contribute to CRT non-response finally.24 Accordingly, the therapeutic target is to minimize the occurrence of such ventricular arrhythmias either through pharmacological therapy or catheter ablation and to maintain biventricular pacing as close to 100% as possible.25
Systolic Dyssynchrony and Diastolic Dyssynchrony in Predicting CRT Response
LV dyssynchrony to predict CRT response has been increasingly investigated in recent years. The technique of phase analysis on gated SPECT MPI to assess mechanical dyssynchrony has been extensively used due to its advantage of high reproducibility and repeatability compared with echocardiography.26 Many studies showed that the presence of LV mechanical dyssynchrony improved response rate and predicted CRT response in a long term.27,28 However, there is a contradiction in predicting CRT response by mechanical dyssynchrony. The baseline systolic PSD and PBW did not show any predictive value of CRT response in two recent studies with relatively large populations.29,30 Till now, majority of studies on LVMD have focused on systolic LVMD rather than diastolic LVMD. Recent studies reported that diastolic LVMD from echocardiography was more frequent than systolic LVMD in both diastolic and systolic heart failure patients and might explain the lack of CRT response despite good systolic synchrony,31,32 which was usually ignored in the past. Chen et al14 found that both diastolic PSD and PBW from SPECT MPI were significantly prolonged in the end-stage renal disease group compared to controls and that diastolic dyssynchrony was present in 65% of subjects compared to 47% of subjects with systolic dyssynchrony. Among patients with coronary heart disease, Fudim et al33 reported that systolic and diastolic left ventricular dyssynchrony from SPECT MPI were associated with adverse outcomes and diastolic dyssynchrony appears to provide incremental predictive value to clinical history, electrical dyssynchrony, and left ventricular function. In our previous study,34 both LV systolic and diastolic dyssynchrony from SPECT MPI had important prognostic values for DCM patients. However, the value of diastolic LVMD from gated MPI in CRT have not been further investigated. Alexander-son-Rosas et al12 reported that CRT improved both systolic and diastolic dyssynchrony values at 6-month follow-up in heart failure patients and suggested its importance in the evaluation of subjects undergoing CRT. They found that diastolic LVMD at baseline was correlated with cardiac functionality, but not with overall favorable clinical outcomes, which might be interfered by the comorbidities of CAD (18.8%) and short period of follow-up(6 months). However, we measured both systolic and diastolic dyssynchrony from gated MPI in DCM patients before CRT and there were significant differences between the CRT response group and the non-response group in our study. More importantly, both baseline values of systolic LVMD and diastolic LVMD were independent predictive factors for CRT response.
Some existing studies about diastolic dyssynchrony may explain its incremental value to systolic dyssynchrony in predicting the CRT response. Schuster et al31 suggested that diastolic abnormalities caused by LBBB included reduced LV filling time, prolonged isovolumetric contraction and relaxation times, altered transmitral filling patterns, and prolonged duration of mitral regurgitation in patients with LV dysfunction. Moreover, Morris-Thurgood et al35 found that diastolic filling abnormalities was crucially important in CRT patients and proposed that part of the benefit of CRT was probably related to better LV filling rather than ventricular systolic resynchronization. The reduction of the ventricular interaction in diastole during LV pacing has also been proposed to be the dominant mechanism by which LV pacing may produce hemodynamic improvement in chronic heart failure patients.36 Therefore, it shows an important clinical implication that the evaluation of diastolic LVMD would change the dilemma of CRT non-response.
The Significance of Diastolic LVMD for the LV Lead Position
Myocardial imaging techniques, including echocardiography,37 CMR38 and gated SPECT MPI39 have been used to recommend LV lead positions for CRT. Friehling et al7 defined a concordant LV lead position as the LV lead placed in the segment with myocardial viability and with or adjacent to the latest mechanical activation, and showed that phase analysis on gated SPECT MPI successfully predicted acute change in LV synchrony and patient outcome after CRT. Boogers et al5 conducted a similar study by phase analysis and showed that pacing at the latest LV activation was associated with improvement in LV reverse remodeling at the 6 months follow up. Moreover, Zhang et.al30 defined the hierarchical recommendations according to contraction delays measured on gated SPECT MPI and concluded that CRT patients with the recommended LV lead site had a higher response rate and better long-term prognosis. In their study, after excluding apical, septal and scarred segments, there were three levels of recommended segments: (1) the optimal recommendation: the latest contracting viable segment; (2) the 2nd recommendation: the viable segments whose contraction delays were within 10 degrees of the optimal recommendation; (3) the 3rd recommendation: the viable segments adjacent to the optimal recommendation when there was no segment in the 2nd recommendation. It was found that the response rates were 66.2% in the patients whose LV lead was in the non-apical or scarred segments, and 27.3% in the patients with LV lead positioning in the apex or scarred segments; by contrast, when the LV leads were placed in the above three recommended levels, there was a similar response rate (76.9%, 76.9% and 73.3%, respectively; P = 0.967). Our study significantly enhances the clinical value of LV dysynchrony in recommending the LV pacing sites for CRT by incorporating systolic LVMD with diastolic LVMD. It showed that in our study the rate of CRT response was 94.4% (17 of 18) in patients whose LV lead was in the segments of both the first three late contraction and the first three late relaxation; by contrast, the rate of CRT response was only 6.7% (1 of 15, P < 0.000) in patients whose LV lead was in the segments with neither the first three late contraction nor the first three late relaxation. Moreover, we found that there were no significant differences between the three patient groups according to the match between the LV lead position and latest contracting or relaxing segments, but the result showed a trend toward different clinical outcomes in Figure 5 (P = 0.152). Based on these findings, we would recommend that LV lead be placed in the segments with both the late contraction and late relaxation. Meanwhile, we should avoid LV placing at the site with neither the late contraction nor late relaxation.
Limitations
First, the population of our retrospective study is relatively small. Second, the definition of CRT response in this study relied on echocardiographic tests to measure the main outcome. Other clinical indices such as quality of life or 6-minutes walking test were not assessed. Third, the follow-up period of our study was relatively short. Fourth, the dyssynchrony data in CRT patients were not compared with those in normal subjects. Besides that, our research is still preliminary, and prospective multicenter studies in a larger population are needed.
CONCLUSIONS
Both systolic LVMD and diastolic LVMD from gated SPECT MPI have important predictive values for CRT response in DCM patients. Pacing at LV segments with both late contraction and late relaxation has potential to increase the CRT response.
Supplementary Material
Acknowledgments
This research was supported by a grant from the American Heart Association(Project Number: 17AIREA33700016, PI: Weihua Zhou) and a new faculty grant from Michigan Technological University Institute of Computing and Cybersystems (PI: Weihua Zhou).
Abbreviations
- DCM
Dilated cardiomyopathy
- CRT
Cardiac resynchronization therapy
- LVMD
Left ventricular mechanical dyssynchrony
- LVEF
Left ventricular ejection fraction
- NYHA
New York Heart Association
- ECG
Electrocardiogram
- NS-VT
Nonsustained ventricular tachycardia
- SPECT
Single photon emission computed tomography
- PSD
Phase standard deviation
- PBW
Phase histogram bandwidth
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s12350-020-02132-1) contains supplementary material, which is available to authorized users.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
Disclosures
Dr. Garcia receives royalties from the sales of Emory Cardiac Toolbox. The terms of this arrangement have been reviewed and approved by the Emory University in accordance with its conflicts of interest practice. None of the other authors, including Cheng Wang, Jianzhou Shi, Jiacheng Ge, Haipeng Tang, Zhuo He, Yanyun Liu, Zhongqiang Zhao, Chunxiang Li, Kai Gu, Xiaofeng Hou, Minglong Chen, Jiangang Zou, Lei Zhou, Ernest V. Garcia, Dianfu Li, Weihua Zhou, has any relevant conflicts of interest.
References
- 1.Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 2002;39:2026–33. [DOI] [PubMed] [Google Scholar]
- 2.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–53. [DOI] [PubMed] [Google Scholar]
- 3.McAlister FA, Ezekowitz J, Hooton N, Vandermeer B, Spooner C, Dryden DM, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: A systematic review. JAMA 2007;297:2502–14. [DOI] [PubMed] [Google Scholar]
- 4.Zhou W, Garcia EV. Nuclear image-guided approaches for cardiac resynchronization therapy (CRT). Curr Cardiol Rep 2016;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boogers MJ, Chen J, van Bommel RJ, Borleffs CJ, Dibbets-Schneider P, van der Hiel B, et al. Optimal left ventricular lead position assessed with phase analysis on gated myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging 2011;38:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ypenburg C, van Bommel RJ, Delgado V, Mollema SA, Bleeker GB, Boersma E, et al. Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol 2008;52:1402–9. [DOI] [PubMed] [Google Scholar]
- 7.Friehling M, Chen J, Saba S, Bazaz R, Schwartzman D, Adelstein EC, et al. A prospective pilot study to evaluate the relationship between acute change in left ventricular synchrony after cardiac resynchronization therapy and patient outcome using a single-injection gated SPECT protocol. Circ Cardiovasc Imaging 2011;4:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verbrugge FH, Verhaert D, Grieten L, Dupont M, Rivero-Ayerza M, De Vusser P, et al. Revisiting diastolic filling time as mechanistic insight for response to cardiac resynchronization therapy. Europace 2013;15:1747–56. [DOI] [PubMed] [Google Scholar]
- 9.Boogers MJ, Chen J, Veltman CE, van Bommel RJ, Mooyaart EA, Al Younis I, et al. Left ventricular diastolic dyssynchrony assessed with phase analysis of gated myocardial perfusion SPECT: A comparison with tissue Doppler imaging. Eur J Nucl Med Mol Imaging 2011;38:2031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu TH, Huang WS, Chen CC, Hung GU, Chen TC, Kao CH, et al. Left ventricular systolic and diastolic dyssynchrony assessed by phase analysis of gated SPECT myocardial perfusion imaging: A comparison with speckle tracking echocardiography. Ann Nucl Med 2013;27:764–71. [DOI] [PubMed] [Google Scholar]
- 11.Fudim M, Dalgaard F, Fathallah M, Iskandrian AE, Borges-Neto S. Mechanical dyssynchrony: How do we measure it, what it means, and what we can do about it. J Nucl Cardiol 2019;29:43–52. [DOI] [PubMed] [Google Scholar]
- 12.Alexanderson-Rosas E, Espinola-Zavaleta N, Garcia EV, Peix A, Massardo T, Pabon LM, et al. Diastolic dyssynchrony assessment by gated myocardial perfusion-SPECT in subjects who underwent cardiac resynchronization therapy. J Nucl Cardiol 2019. 10.1007/s12350-019-01845-2. [DOI] [PubMed] [Google Scholar]
- 13.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008;29:270–6. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Kalogeropoulos AP, Verdes L, Butler J, Garcia EV. Left-ventricular systolic and diastolic dyssynchrony as assessed by multi-harmonic phase analysis of gated SPECT myocardial perfusion imaging in patients with end-stage renal disease and normal LVEF. J Nucl Cardiol 2011;18:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White JA, Yee R, Yuan X, Krahn A, Skanes A, Parker M, et al. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol 2006;48:1953–60. [DOI] [PubMed] [Google Scholar]
- 16.Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J 2007;153:105–12. [DOI] [PubMed] [Google Scholar]
- 17.Sciagra R, Giaccardi M, Porciani MC, Colella A, Michelucci A, Pieragnoli P, et al. Myocardial perfusion imaging using gated SPECT in heart failure patients undergoing cardiac resynchronization therapy. J Nucl Med 2004;45:164–8. [PubMed] [Google Scholar]
- 18.Adelstein EC, Tanaka H, Soman P, Miske G, Haberman SC, Saba SF, et al. Impact of scar burden by single-photon emission computed tomography myocardial perfusion imaging on patient outcomes following cardiac resynchronization therapy. Eur Heart J 2011;32:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riedlbauchova L, Brunken R, Jaber WA, Popova L, Patel D, Lanska V, et al. The impact of myocardial viability on the clinical outcome of cardiac resynchronization therapy. J Cardiovasc Electrophysiol 2009;20:50–7. [DOI] [PubMed] [Google Scholar]
- 20.Lalonde M, Birnie D, Ruddy TD, deKemp RA, Beanlands RS, Wassenaar R, et al. SPECT gated blood pool phase analysis of lateral wall motion for prediction of CRT response. Int J Cardiovasc Imaging 2014;30:559–69. [DOI] [PubMed] [Google Scholar]
- 21.Ypenburg C, Schalij MJ, Bleeker GB, Steendijk P, Boersma E, Dibbets-Schneider P, et al. Impact of viability and scar tissue on response to cardiac resynchronization therapy in ischaemic heart failure patients. Eur Heart J 2007;28:33–41. [DOI] [PubMed] [Google Scholar]
- 22.Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, de Roos A, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation 2006;113:969–76. [DOI] [PubMed] [Google Scholar]
- 23.Murphy RT, Sigurdsson G, Mulamalla S, Agler D, Popovic ZB, Starling RC, et al. Tissue synchronization imaging and optimal left ventricular pacing site in cardiac resynchronization therapy. Am J Cardiol 2006;97:1615–21. [DOI] [PubMed] [Google Scholar]
- 24.Sieniewicz BJ, Gould J, Porter B, Sidhu BS, Teall T, Webb J, et al. Understanding non-response to cardiac resynchronisation therapy: Common problems and potential solutions. Heart Fail Rev 2019;24:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haugaa KH, Dan GA, Iliodromitis K, Lenarczyk R, Marinskis G, Osca J, et al. Management of patients with ventricular arrhythmias and prevention of sudden cardiac death-translating guidelines into practice: Results of the European Heart Rhythm Association survey. Europace 2018;20:f249–53. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Faber TL, Patel Z, Folks RD, Cheung AA, Garcia EV, et al. An automatic alignment tool to improve repeatability of left ventricular function and dyssynchrony parameters in serial gated myocardial perfusion SPECT studies. Nucl Med Commun 2013;34:124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beela AS, Unlu S, Duchenne J, Ciarka A, Daraban AM, Kotrc M, et al. Assessment of mechanical dyssynchrony can improve the prognostic value of guideline-based patient selection for cardiac resynchronization therapy. Eur Heart J Cardiovasc Imaging 2019;20:66–74. [DOI] [PubMed] [Google Scholar]
- 28.Tayal B, Gorcsan J 3rd, Bax JJ, Risum N, Olsen NT, Singh JP, et al. Cardiac resynchronization therapy in patients with heart failure and narrow QRS complexes. J Am Coll Cardiol 2018;71:1325–33. [DOI] [PubMed] [Google Scholar]
- 29.Peix A, Karthikeyan G, Massardo T, Kalaivani M, Patel C, Pabon LM, et al. Value of intraventricular dyssynchrony assessment by gated-SPECT myocardial perfusion imaging in the management of heart failure patients undergoing cardiac resynchronization therapy (VISION-CRT). J Nucl Cardiol 2019. 10.1007/s12350-018-01589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Qian Z, Tang H, Hua W, Su Y, Xu G, et al. A new method to recommend left ventricular lead positions for improved CRT volumetric response and long-term prognosis. J Nucl Cardiol 2019. 10.1007/s12350-019-01735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuster I, Habib G, Jego C, Thuny F, Avierinos JF, Derumeaux G, et al. Diastolic asynchrony is more frequent than systolic asynchrony in dilated cardiomyopathy and is less improved by cardiac resynchronization therapy. J Am Coll Cardiol 2005;46:2250–7. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Kurrelmeyer KM, Torre-Amione G, Nagueh SF. Systolic and diastolic dyssynchrony in patients with diastolic heart failure and the effect of medical therapy. J Am Coll Cardiol 2007;49:88–96. [DOI] [PubMed] [Google Scholar]
- 33.Fudim M, Fathallah M, Shaw LK, Liu PR, James O, Samad Z, et al. The prognostic value of diastolic and systolic mechanical left ventricular dyssynchrony among patients with coronary heart disease. JACC Cardiovasc Imaging 2019;12:1215–26. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Tang H, Zhu F, Jiang Z, Shi J, Zhou Y, et al. Prognostic value of left-ventricular systolic and diastolic dyssynchrony measured from gated SPECT MPI in patients with dilated cardiomyopathy. J Nucl Cardiol 2018. 10.1007/s12350-018-01468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris-Thurgood JA, Turner MS, Nightingale AK, Masani N, Mumford C, Frenneaux MP. Pacing in heart failure: Improved ventricular interaction in diastole rather than systolic re-synchronization. Europace 2000;2:271–5. discussion 6. [DOI] [PubMed] [Google Scholar]
- 36.Bleasdale RA, Turner MS, Mumford CE, Steendijk P, Paul V, Tyberg JV, et al. Left ventricular pacing minimizes diastolic ventricular interaction, allowing improved preload-dependent systolic performance. Circulation 2004;110:2395–400. [DOI] [PubMed] [Google Scholar]
- 37.Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O’Halloran D, Elsik M, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: The TARGET study: A randomized, controlled trial. J Am Coll Cardiol 2012;59:1509–18. [DOI] [PubMed] [Google Scholar]
- 38.Kockova R, Sedlacek K, Wichterle D, Sikula V, Tintera J, Jansova H, et al. Cardiac resynchronization therapy guided by cardiac magnetic resonance imaging: A prospective, single-centre randomized study (CMR-CRT). Int J Cardiol 2018;270:325–30. [DOI] [PubMed] [Google Scholar]
- 39.Sommer A, Kronborg MB, Norgaard BL, Poulsen SH, Bouchelouche K, Bottcher M, et al. Multimodality imaging-guided left ventricular lead placement in cardiac resynchronization therapy: A randomized controlled trial. Eur J Heart Fail 2016;18:1365–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


