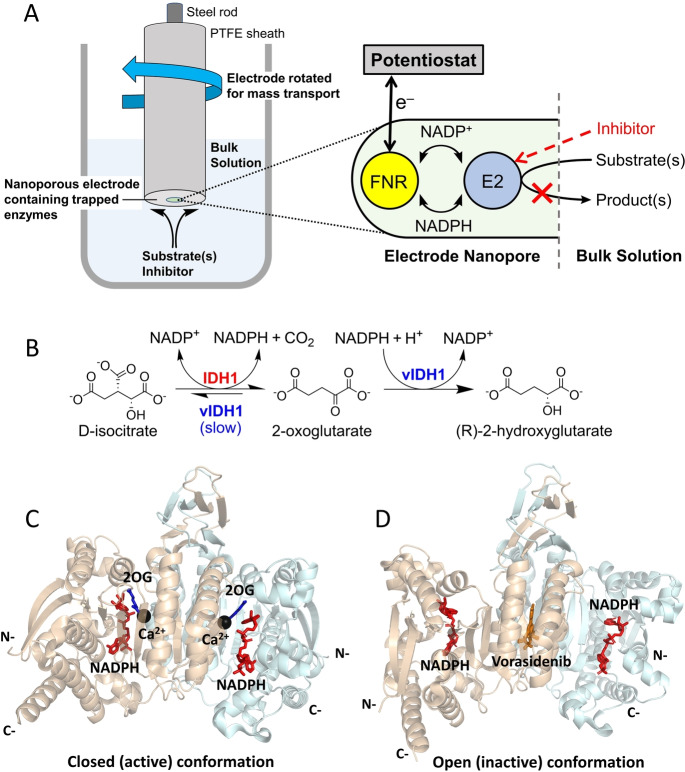
Figure 1.
The e‐Leaf can be used to study dehydrogenase catalysis. (A) Setup of the e‐Leaf when used as an analytical tool. E2 is an NADP(H)‐dependent dehydrogenase. In this case, a rotating‐disc electrode is used to optimise substrate/inhibitor mass transport. The expanded inset shows a cartoon indicating the sequence of tightly‐channeled electrochemical information flow. (B) Reactions of wildtype IDH1 and 2HG‐producing IDH1 variants (vIDH1). Note that the variant IDH1 enzymes catalyse the wildtype reaction (isocitrate oxidation), but at a reduced rate.[ 12 , 13 , 14 ] (C and D) Views from crystal structures of IDH1 R132H. (C) The proposed closed (active) conformation with 2OG, NADP(H), and inhibitory Ca2+ (substituting for Mg2+) bound at the active site of each monomer (PDB: 4KZO). [15] (D) The proposed open (inactive) conformation with one molecule of an inhibitor, Vorasidenib, bound at the dimer interface and a molecule of NADP(H) bound at the active site of each monomer (PDB: 6ADG). [16]