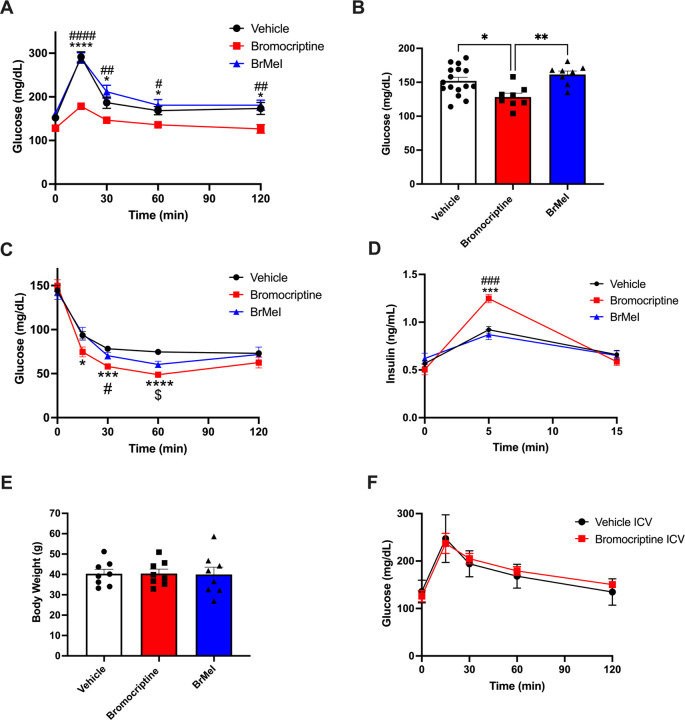
Figure 6. Tandem targeting of central and peripheral dopaminergic targets is required for treating dysglycemia in vivo.
(A) Western diet-fed dysglycemic mice were systemically treated with bromocriptine (10 mg/kg i.p., in red), BrMeI (10 mg/kg i.p., in blue), or vehicle (i.p., in black). Oral glucose tolerance testing (OGTT, 2g/kg glucose) revealed significant effects of drug treatment (p < 0.0001) on improvement in glucose tolerance driven by bromocriptine (n = 8); BrMeI (n = 8) did not significantly modify glucose tolerance (p > 0.05) versus vehicle (n = 16). *<0.05 bromocriptine versus vehicle; #<0.05, ##<0.01, ####<0.0001 bromocriptine versus BrMeI. (B) Bromocriptine-treated mice (n = 8) exhibited significantly lower fasting blood glucose compared to BrMeI (p = 0.0035, n = 8) or vehicle (p = 0.0163, n = 16). *<0.05, **<0.01. (C) Insulin tolerance testing (ITT) showed that bromocriptine treatment (10 mg/kg i.p., in red; n = 8) led to significant improvements in insulin sensitivity versus vehicle (i.p., n = 8), with smaller improvements produced by BrMeI (10 mg/kg i.p., n = 8). *<0.05, ***<0.001, ****<0.0001 bromocriptine versus vehicle; #<0.05 bromocriptine versus BrMeI; $<0.05 BrMeI versus vehicle. (D) Bromocriptine treatment also significantly enhanced plasma insulin levels during the ITT compared to BrMeI (p = 0.0003) or vehicle (p = 0.0002) groups. ***<0.001 bromocriptine versus vehicle, ###<0.001 bromocriptine versus BrMeI. (E) There was no significant effect of bromocriptine or BrMeI on body weight versus vehicle treatment in Western diet-fed wild-type mice (p > 0.05) (n=8/group). (F) I.c.v. administration of bromocriptine (10 mg/kg, in red) in Western diet-fed dysglycemic wild-type mice. OGTT (2 g/kg glucose) did not significantly modify glucose tolerance versus i.c.v. vehicle treatment (in black) (n=8/group; p>0.05). All data represented as mean ± SEM.