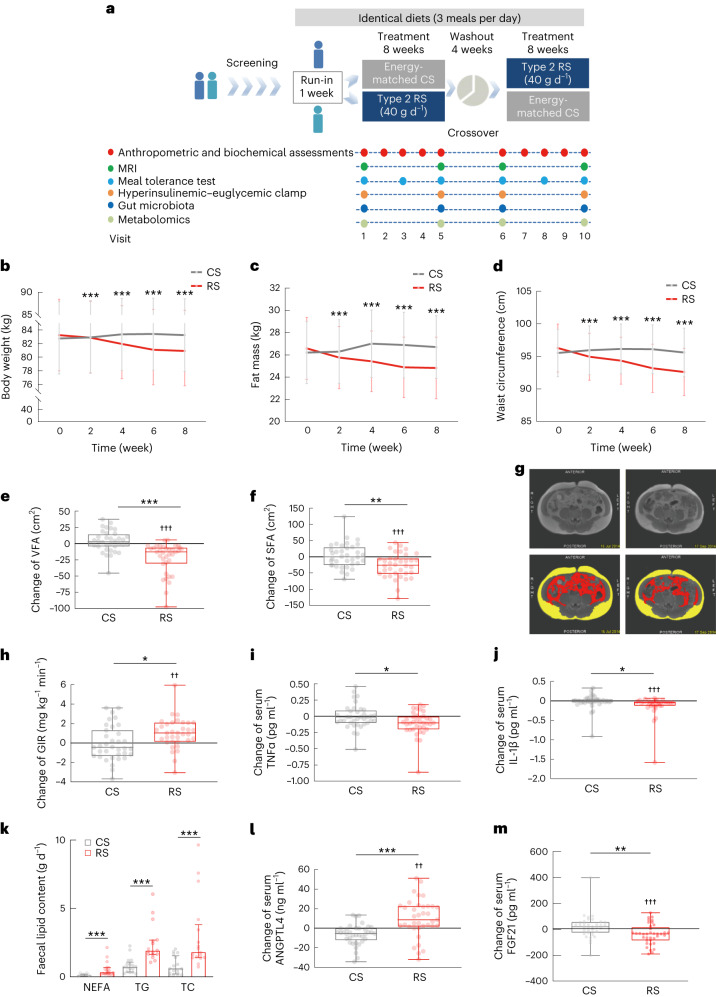
Fig. 1. Alleviation of obesity after the 8-week RS intervention in individuals with excess body weight.
a, Diagram of the clinical trial. After enrolment, randomization and run-in period, participants consumed either RS or CS alternately and separated by a washout period. During the whole trial, all participants were provided with identical diets. The assessments at each visit are displayed in the diagram. b–d, RS intervention significantly reduced body weight (b), fat mass (c) and waist circumference (d). e,f, Change of VFA and SFA evaluated by MRI. g, Representative abdominal MRI of participants before (left) and after (right) the 8-week RS intervention. Raw (top) and marked (bottom) MRI at navel level. Yellow represents SFA and red represents VFA. h, Change of GIR evaluated by hyperinsulinemic–euglycemic clamp. i, Change of serum TNFα levels. j, Change of serum IL-1β levels. k, Daily faecal lipid excretion, including NEFA, TG and TC after the 8-week interventions with RS or CS. l, Change of serum ANGPTL4 levels. m, Change of serum FGF21 levels. n = 37 individuals (b–d,j,l,m), n = 36 individuals (e,f,i), n = 35 individuals (h) and n = 17 individuals (k) for either RS or CS. Analysis of covariance (ANCOVA) adjusted by baseline value was used for comparison between RS and CS at each visit (b–d). Data are shown as mean (95% confidence interval (CI)). ***P < 0.001. Data are shown as median with IQR (k). Nonparametric Wilcoxon rank-sum test was used to evaluate the significance between the two interventions. ***P < 0.001. Data are shown as box-and-whisker plots (e,f,h–j,l,m). Box plot, median and quartiles; whiskers, data range. *P = 0.025, 0.014 and 0.046 (h–j), **P = 0.004 and 0.002 (f,m), ***P < 0.001 for the between-group difference assessed by the linear mixed model adjusted for intervention order. ††P = 0.003 and 0.002 (h,l). †††P < 0.001 for the within-group change by mixed linear model adjusted for intervention followed by Bonferroni’s test.