Abstract
Resection of gliomas in or close to motor areas is at high risk for morbidity and development of surgery-related deficits. Navigated transcranial magnetic stimulation (nTMS) including nTMS-based tractography is suitable for presurgical planning and risk assessment. The aim of this study was to investigate the association of postoperative motor status and the spatial relation to motor eloquent brain tissue in order to increase the understanding of postoperative motor deficits.
Patient data, nTMS examinations and imaging studies were retrospectively reviewed, corticospinal tracts (CST) were reconstructed with two different approaches of nTMS-based seeding. Postoperative imaging and nTMS-augmented preoperative imaging were merged to identify the relation between motor positive cortical and subcortical areas and the resection cavity.
38 tumor surgeries were performed in 36 glioma patients (28.9% female) aged 55.1 ± 13.8 years. Mean distance between the CST and the lesion was 6.9 ± 5.1 mm at 75% of the patient-individual fractional anisotropy threshold and median tumor volume reduction was 97.7 ± 11.6%. The positive predictive value for permanent deficits after resection of nTMS positive areas was 66.7% and the corresponding negative predictive value was 90.6%. Distances between the resection cavity and the CST were higher in patients with postoperative stable motor function. Extent of resection and distance between resection cavity and CST correlated well.
The present study strongly supports preoperative nTMS as an important surgical tool for preserving motor function in glioma patients at risk.
Keywords: Navigated transcranial magnetic stimulation, Ntms motor mapping, Neuronavigation-neurooncology, Functional imaging, Glioma surgery
Abbreviations
- APB –
abductor pollicis brevis muscle
- ADM –
abductor digiti minimi muscle
- CI –
confidence interval
- CST –
corticospinal tract
- CTD –
cavity to tract distance (distance between resection cavity and pyramidal tract on axial MRI)
- DES –
direct electrical stimulation
- DTI –
diffusion tensor imaging
- EMG –
electromyogram
- EOR –
extent of resection
- FA –
fractional anisotropy
- FAT –
fractional anisotropy threshold
- FT –
fibertracking
- IOM –
intraoperative monitoring
- IQR –
interquartile range
- LTD -
lesion to tract distance (distance between the lesion and pyramidal tract on axial MRI)
- MRI –
magnetic resonance imaging
- rMT –
resting motor threshold
- ROI –
region of interest
- SD –
standard deviation
- TE –
echo time
- TR –
repetition time
1. Introduction
The prognosis of patients with gliomas is affected by individual demographic, tumor- and treatment-related factors. Two factors can be influenced by the operating neurosurgeon: the extent of resection and the patient's postoperative functional status [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10]]. In order to avoid permanent neurological deficits, several functional imaging tools including functional MRI, navigated transcranial magnetic stimulation (nTMS), diffusion tensor imaging tractography (DTI) and intraoperative imaging have been established in order to achieve a maximum safe resection [[11], [12], [13], [14], [15]]. Among above-mentioned functional imaging modalities, nTMS has been advocated to be a valuable tool for preoperative brain mapping, especially for the evaluation of the motor system. The advantages of nTMS based presurgical planning are widely accepted and nTMS data are superior to other non-invasive functional imaging modalities [14,[16], [17], [18], [19], [20], [21], [22], [23], [24]]. The combination of nTMS motor mapping and tractography allows patient-individual visualization of cortical and subcortical motor eloquent brain [[25], [26], [27]].
Based upon nTMS motor mapping and fibertracking, the risk for developing new motoric deficits can be estimated. General risk factors for postoperative deterioration in motor function are the distance between the lesion and the pyramidal tract (LTD) [[28], [29], [30], [31], [32]] and the resection of preoperatively identified nTMS motor positive areas. Both nTMS motor positive spots within the primary motor cortex and even within non-primary motor areas should be preserved in order to preserve the patients’ motor status [[28], [29], [30],33,34]. The aim of the present study was to provide information and to externally confirm previously published results concerning the association of postoperative motor status in motor-eloquent glioma patients in relation to the results of nTMS motor mapping results and nTMS based tractography. We additionally investigated several approaches of reconstructing the pyramidal tract in order to counterbalance some common issues of DTI-fibertracking [15,35]. The primary outcome of our study was deterioration of motor function after surgery within the first days after surgery and a residual deficit on follow-up visit. Secondary outcome was the extent of resection on postoperative MRI.
2. Methods
2.1. Compliance with Ethical standards
The study design was approved by the Institutional Review Board of Paracelsus Medical University Nuremberg (Registration numbers IRB-2020-022 and IRB-2022-012). All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments. Informed consent for study participation was waived due to the retrospective study design and statistical analysis of anonymized data.
2.2. Patient cohort
Patients treated between April 2016 and June 2021 at our institution were retrospectively reviewed. Inclusion criteria were age over 18 years, preoperative nTMS and DTI for evaluation of the motor system, histologically confirmed glioma CNS WHO grade 2–4, availability of pre- and postoperative MR imaging and surgery with the aim to achieve maximum safe resection. Patients with LTD >20 mm and one patient with postoperative subcortical ischemia on diffusion MRI likely for causing a new motor deficit were excluded from the study. We further excluded four patients who died from medical complications before discharge from hospital. All surgical procedures were conducted under general anesthesia in a microsurgical 5-ALA fluorescence guided approach with the nTMS data integrated into the neuronavigation system. Based upon clinical examination, preoperative motor deficits were assessed as “no deficit”, “mild” (disruption of fine motor skills or protonation in arm drift test, facial palsy), “moderate” (incomplete hemiparesis) and “severe” (disability to lift one extremity from surface or plegia of one or more extremities). Pre- and postoperative neurological examinations were performed by neurosurgeons. A permanent deficit was defined as residual surgery-related deficit which occurred in the immediate postoperative phase and did not return to the preoperative status within at least six weeks of follow-up. Parts of this cohort have been published recently [36].
2.3. Imaging and navigated brain stimulation
All patients underwent pre- and postoperative MR-imaging including a 3D post-contrast T1 weighted sequence. Preoperative imaging additionally included diffusion tensor imaging (DTI with 32 gradient directions). All nTMS examinations were conducted with the NexStim NBS 5 system (NexStim Oy, Helsinki, Finland, Fig. 1A) and the workflow followed recently published guidelines [37]. Slight modifications were made and described in the following.
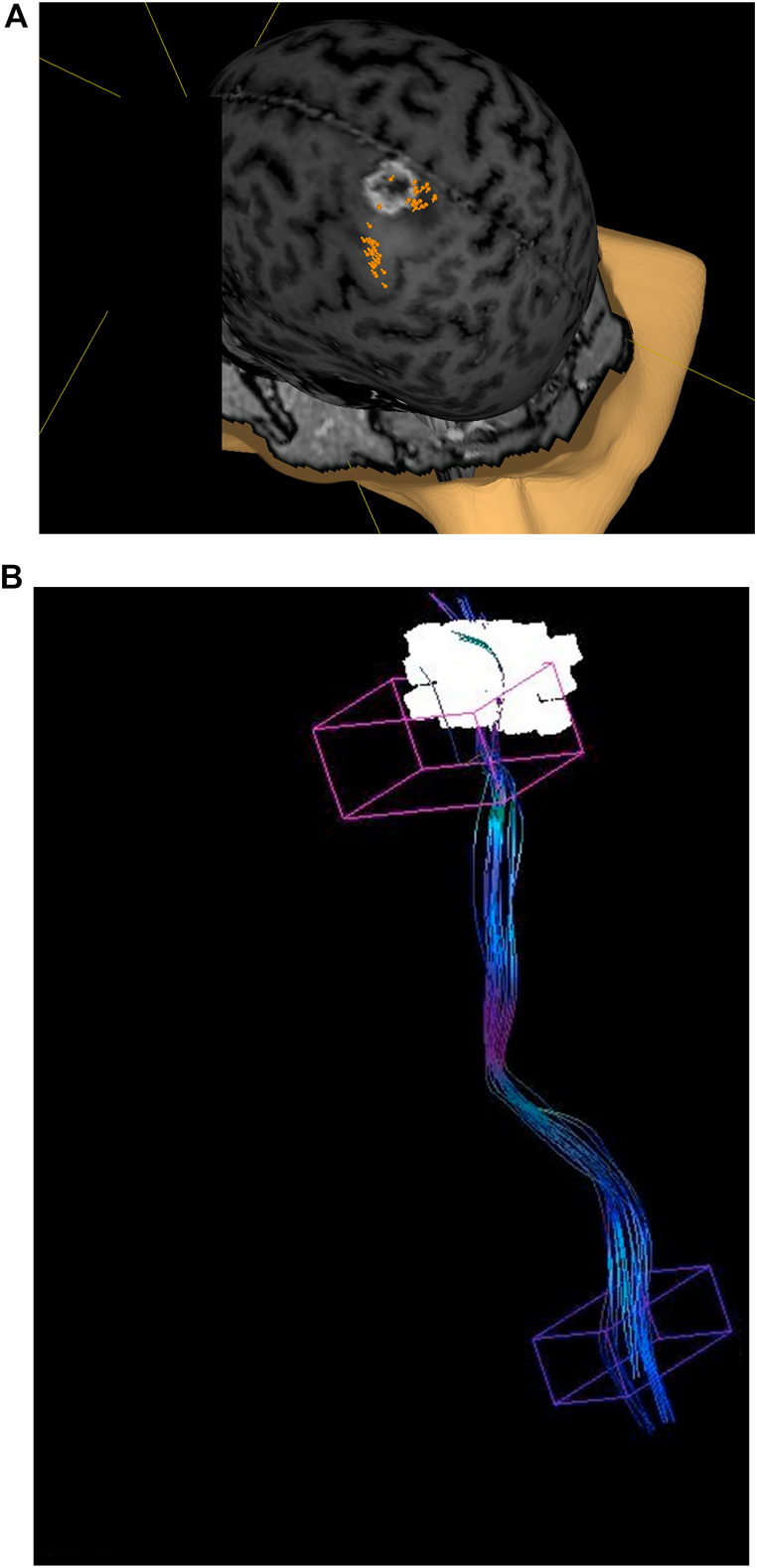
Fig. 1.
Identifying the hand motor area with nTMS. Motor positive spots are displayed in orange (A). Seeding regions for fibertracking (B), with the nTMS-based ROI displayed in white and the anatomical ROI in pink. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Ambu Neuroline 700 surface electrodes were used in all cases (Ambu, Denmark) and attached to the target muscles in a belly-tendon-fashion. The ground electrode was placed at the patient's elbow.
A minimum of two intrinsic hand muscles (abductor pollicis brevis (APB), abductor digiti minimi (ADM) was monitored for the upper extremity. Lower limb muscles (tibialis anterior and/or gastrocnemius muscle) were mapped for tumors close to the anatomical leg motor area.
The first round of mapping was conducted to determine the hand motor hotspot at a default stimulation intensity of 30–35% stimulator output. Stimulation intensity was increased if no MEPs could be elicited. The first round of mapping was started at the omega-shaped portion of the precentral gyrus. If MEPs from the hand muscles could be registered, the stimulation site which produced the greatest peak-to-peak-amplitude was stimulated several times to ensure reproducibility of MEPs. That spot was considered the hand motor hotspot and was used for determination of the resting motor threshold.
The patient-individual resting motor threshold (rMT) was determined using the NBS system's threshold hunting algorithm. Mapping of the cortical representations of the upper extremity muscles was performed at 110–120% rMT (with a standard of 110%rMT and intensity was only increased if patients had difficulties in muscle relaxation).
The leg area was mapped with a stimulation intensity of at least 110% of the upper-extremity rMT. The primary motor cortex of the upper extremity and the peritumoral cortex were mapped in all cases. Each examination was analyzed post-hoc for positive muscle responses defined as MEPs with amplitudes greater than 50 μV and with latencies within a range of 10–30 ms. nTMS positive spots outside the precentral gyrus were additionally evaluated for the electrical field current at the corresponding extremity's motor hotspot. If the corresponding electric field current at the motor hotspot reached the electric field current of the patient's motor threshold, these stimulations were classified as false positive due to possible indirect stimulation of the primary motor cortex and subsequently excluded from the analysis.
2.4. DTI-tractography
The nTMS-positive spots were exported via the standard DICOM format for tractography using the Medtronic StealthStation S8 StealthViz/StealthDTI (Medtronic Inc, Louisville, CO, USA) module. In the post-hoc analysis, the nTMS spots were enlarged to a diameter of 6 mm and served as a cortical region of interest (ROI). A second standard anatomy-based cubic ROI was placed within the caudal pons. Each corticospinal tract (CST) was visualized as follows: Tractography was performed at 75% and 50% of the patient-individual maximum fractional anisotropy (fractional anisotropy threshold – FAT). Minimum fiber length was set to 110 mm and the maximum directional change to 60°. Fibers which clearly did not belong to the corticospinal tract were removed. In addition to the above-mentioned approach, a nTMS assisted anatomical CST reconstruction was performed. A cubic ROI was placed slightly below the nTMS-positive motor area and served as an alternative (sub-) cortical seeding region (Fig. 1B). Software settings were identical to the settings described above. The nTMS assisted anatomical CST reconstruction was conducted as it was not possible to visualize the CST in all patients by purely using the enlarged nTMS spots as a cortical seeding region in our cohort. However, we did not aim to compare different approaches of tractography in this study.
The closest distance to the lesion (lesion-to-tract-distance (LTD)) measured on the axial plane were recorded for statistical analysis. For reasons of clarity, only the statistical evaluation and results of the purely nTMS-based DTI fibertracking is displayed in the text unless mentioned otherwise. The results of the nTMS-assisted anatomical fibertracking are shown in the tables and figures.
2.5. Data- and imaging analysis
Pre- and postoperative tumor volume was assessed using the Medtronic StealthStation S8 (Medtronic Inc, Louisville, CO, USA) software. A neuroradiologist evaluated all postoperative MRI examinations for residual tumor tissue. The post-contrast T1 studies were used for volumetric assessment in high grade glioma patients and T2/FLAIR images were used in non-enhancing tumors. Pre- and postoperative MRI as well as nTMS positive areas and reconstructions of the pyramidal tracts were merged using the StealthViz/StealthDTI and the software's inbuilt automatic fusion algorithm. All fusion results were checked for correct fusion and manually corrected if necessary. nTMS spots were classified as resected if they projected into the resection cavity.
The minimum distance between the resection cavity and the CST-reconstructions was measured on the axial plane (cavity to tract distance (CTD)) We classified the fused images as intersection of the CST and the resection cavity if the distance between the resection cavity and any CST-reconstruction was 0 mm.
Pre- and postoperative imaging analyses were subsequently compared to the patients’ preoperative and postoperative status. Postoperative motor status was assessed as stable, transient (surgery-related) deficit or permanent (surgery-related) deficit.
Clinical postoperative status was analyzed for nTMS spots at tumor margins on preoperative imaging, resection of nTMS positive spots and intersection with CST reconstructions as well as differences in LTD, CTD measurements. Resected nTMS spots outside the precentral gyrus were additionally analyzed.
The extent of resection was correlated with LTD and CTD measurements as well as with resected nTMS spots, intersection with the CST and the postoperative clinical status.
We further conducted a subgroup analysis of Glioblastoma patients with similar analyses.
2.6. Statistical analysis
Statistical analysis was carried out using IBM SPSS® Version 27 for Windows 10 (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp).
Metric variables are presented as means and standard deviations (±SD) for age and distances and as medians and interquartile ranges (±IQR) for tumor volumes. Categorial variables are presented as absolute number (n) and percentage (%).
Fisher Exact Tests and Fisher-Freeman-Halton-Tests were applied for categorial variables. For significant results, Cramer's V is provided as a measure of effect size.
For categorial variables, positive and negative predictive values were calculated.
The Mann-Whitney-U-Test and Kruskal-Wallis-Tests were conducted for nonparametric testing of continuous variables. Cohen's d was calculated to measure effect size for non-parametric tests in significant results.
Correlation analyses were performed using Spearman's correlation with Spearman's Rho (rs) and p-values displayed. A p-value <0.05 was considered significant in two-tailed testing.
3. Results
3.1. Patient cohort
38 tumor surgeries were performed in 36 patients. Two patients were operated on for primary and recurrent tumors. 11 patients (28.9%) were female and mean age at surgery was 55.1 ± 13.8 years. 16 (42.1%) patients presented with motor deficits on admission of whom 7 (18.4%) were classified as mild, 7 (18.4%) as moderate and 2 (5.3%) as severe. Table 1 summarizes the baseline data of the patient cohort.
Table 1.
Overview over the patient cohort.
| Item | N (%) | Mean ± SD | Median ± IQR |
|---|---|---|---|
| Demography | |||
| age | 55.1 ± 13.8 | ||
| female | 11 (28.9%) | ||
| Clinical evaluation | |||
| no motor deficit | 22 (57.9%) | ||
| hemiparesis | 5 (13.2%) | ||
| isolated upper limb paresis | 5 (13.2%) | ||
| isolated lower limb paresis | 1 (2.6%) | ||
| disruption of fine motor skills | 4 (10.5%) | ||
| facial palsy | 1 (2.6%) | ||
| Tumor-specific characteristics | |||
| tumor volume (cm³) | 24.0 ± 41.5 | ||
| recurrence |
9 (23.7%) | ||
| WHO grade 2 | 5 (13.2%) | ||
| WHO grade 3 | 7 (18.4%) | ||
| WHO grade 4 | 26 (68.4%) | ||
| perilesional edema | 33 (86.8%) | ||
| lefthemispheric | 14 (36.8%) | ||
| tumor location | |||
| frontal outside gyrus precentralis | 15 (39.5%) | ||
| gyrus precentralis | 8 (21.1%) | ||
| gyrus postcentralis | 5 (13.2%) | ||
| parietal outside gyrus postcentralis | 5 (13.2%) | ||
| temporal | 5 (13.2%) | ||
| Outcome | |||
| postoperative motor function | |||
| stable | 27 (71.1%) | ||
| transient deficit | 4 (10.5%) | ||
| permanent deficit | 7 (18.4%) | ||
| extent of resection | |||
| residual tumor volume (cm³) | 0.3 ± 3.0 | ||
| extent of resection (%) | 97.7 ± 11.6 | ||
3.2. nTMS examination and tractography
Navigated brain stimulation was well-tolerated in all patients and no adverse events occurred in the cohort. MEPs could be obtained in all patients. Mean resting motor threshold (rMT) was 32.8 ± 11.6% of the system's stimulator output. nTMS positive spots at tumor margin were present in 12 cases (31.6%) and associated with preoperative motor deficits (p = 0.014). The closest distance between the CST-fibers and the lesion (lesion-to-tract-distance - LTD) measured 6.9 ± 5.1 mm at 75% FAT. In five cases (12.8%), the nTMS-based approach did not visualize any fibers which clearly belonged to the CST. nTMS-assisted anatomical fibertracking could be conducted successfully in all patients of the study. Mean LTD was 6.8 ± 5.6 mm at 75% for the nTMS-assisted anatomical approach. LTDs between both approaches for tractography correlated well (rs = 0.79, p < 0.001 at both 75% and 50% FAT).
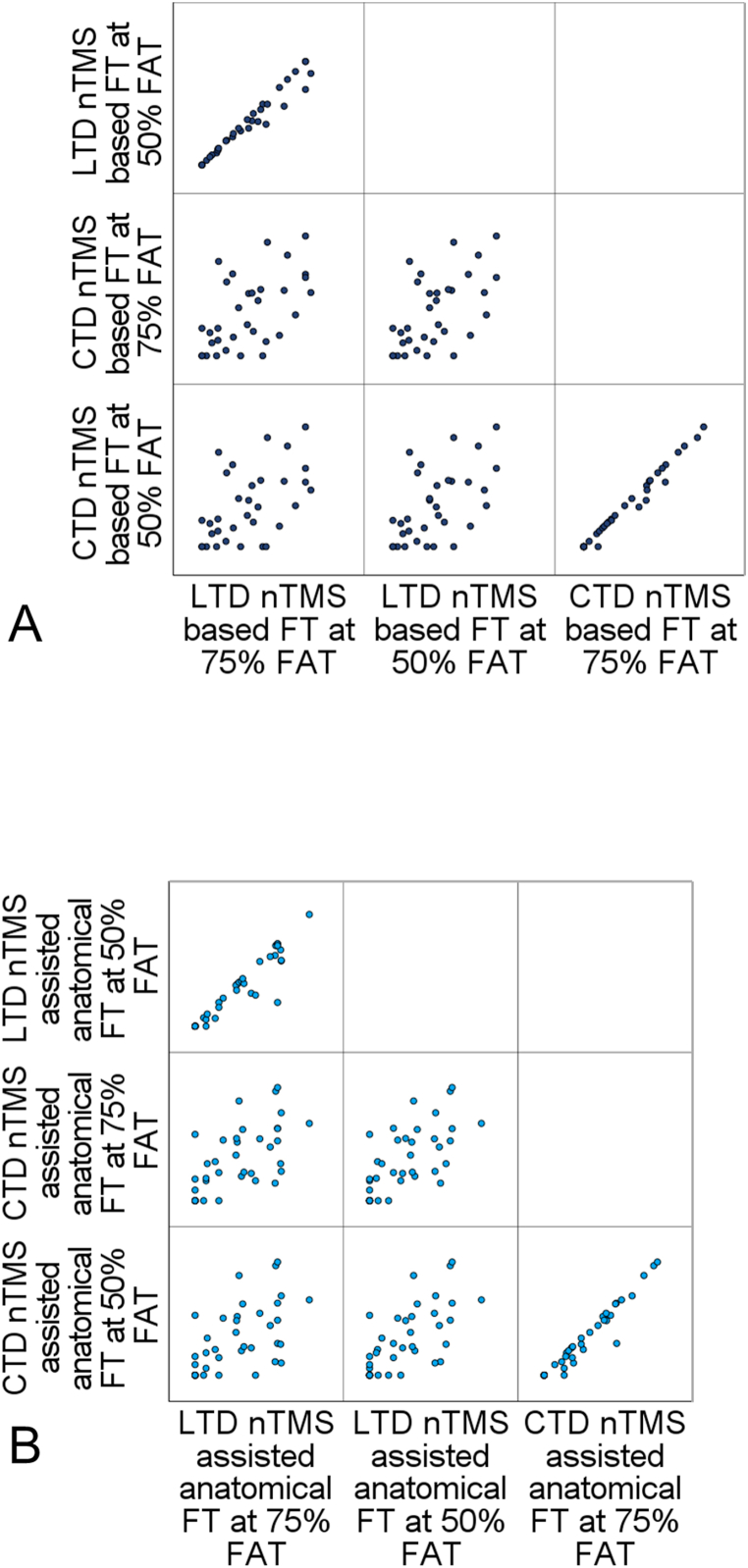
Mean distance between the resection cavity and the corticospinal tract was 9.7 ± 8.1 mm at 75% FAT. For all CST-reconstruction-approaches, LTD and CTD showed significant correlations. CTDs for the different CST-reconstructions and FA-values showed strong correlations. The results of the correlation analyses are outlined in Fig. 2A and B. Intersection of the reconstructed CSTs with the resection cavity was observed in 10 cases (26.3%).
Fig. 2.
Results of the correlation analyses of LTD and CTD measurements for nTMS-based fibertracking (A) and nTMS assisted anatomical fibertracking (B). All correlation analyses revealed significant correlations (all p < 0.01) with a correlation coefficient rs > 0.5.
3.3. Functional outcome in relation to nTMS and tractography
11 patients (28.9%) developed new motor deficits or aggravation of a preoperative motor deficit at discharge from hospital. 4 patients (10.5%) recovered until the follow-up visit and 7 (18.4%) had a permanent surgery-related moderate motor deficit. No patient suffered from a permanent surgery-related severe motor deficit and only two patients (9.1%) without a preoperative tumor-induced deficit suffered from a permanent surgery-related motor deterioration.
nTMS positive spots at the tumor margin were visualized in 12 cases (31.6%). In these cases, surgery-related motor decline occurred in 6 cases and the new deficit remained in 4 patients (p = 0.017, Cramer's V = 0.45). This resulted in a positive predictive value of 50% for any motor deterioration and 33.3% for permanent deficits if nTMS positive spots were present at tumor margins. Vice-versa the negative predictive value for stable motor function was 88.5% if the tumor did not infiltrate the nTMS positive area.
Only two patients with nTMS positive spots at the tumor margin outside the precentral gyrus as identified by nTMS developed a permanent motor deficit.
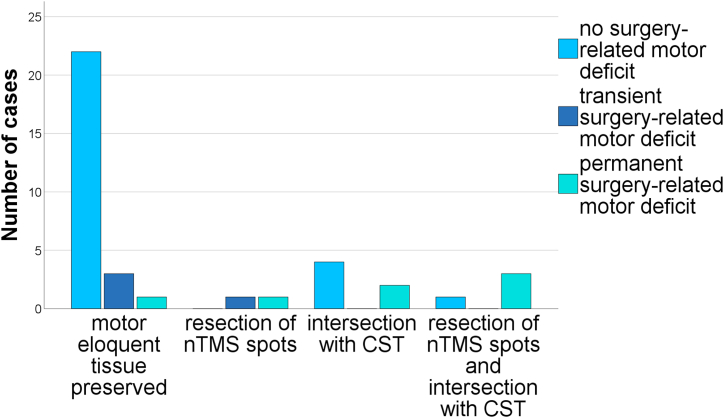
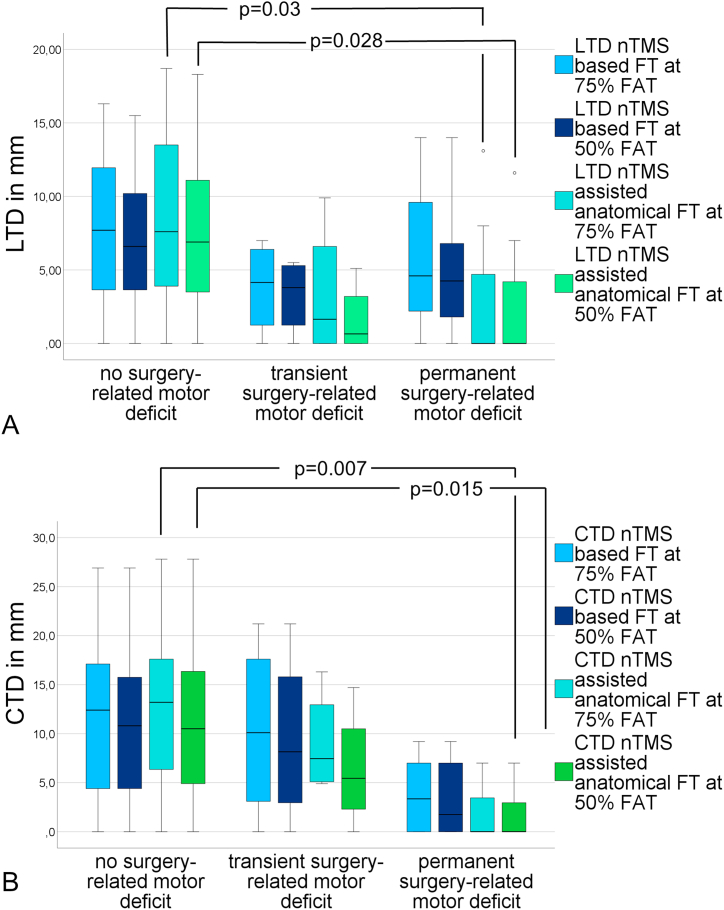
nTMS spots had to be resected in 6 cases (15.8%) and this was strongly associated with infiltration to nTMS-motor-positive gyri as observed in preoperative mapping (p < 0.001, Cramer's V = 0.64). Resection of nTMS positive spots lead to permanent surgery-related deficits (p = 0.003 Cramer's V = 0.57). The positive predictive value for permanent deficits after resection of nTMS positive areas was 66.7% and the corresponding negative predictive value was 90.6% (Table 2, Fig. 3). Correspondingly, intersection of the CST and the resection cavity was associated with permanent motor deficits (p = 0.012, Cramer's V = 0.5). The resection of nTMS positive spots and subcortical white matter pathways (i.e. intersection of the CST and the resection cavity) could explain all but one permanent deficits. The greatest CTD to develop a permanent surgery-related deficit was 9.2 mm at 75%FAT, regardless of resection of nTMS positive spots. In the analysis of subcortical fiber pathways, we observed shorter LTDs in the anatomical approach if patients developed postoperative permanent motor deficits (Fig. 4 A, B).
Table 2.
Positive and negative predictive values for motor outcome in relation to motor eloquent tissue.
| transient deficit | permanent deficit | PPV | NPV | p | |
|---|---|---|---|---|---|
| nTMS positive spots at tumor margin | 3/12 | 4/12 | 33.3% | 88.5% | 0.017 |
| resection of nTMS positive spots | 1/6 | 4/6 | 66.7% | 90.6% | 0.003 |
| intersection with CST | 0/10 | 5/10 | 50% | 92.9% | 0.012 |
Fig. 3.
Functional outcome in relation to resected motor eloquent tissue indicating increasing morbidity with both cortical and subcortical motor areas resected (p=0.002, Cramer’s V=0.5).
Fig. 4.
Analysis of LTD (A) and CTD (B) measurements in relation to the postoperative motor status of the patients with only partly significant results (LTD: 75%FAT nTMS assisted anatomical fibertracking, p = 0.03, Cohen's d = 0.37, 50%FAT nTMS assisted anatomical fibertracking p = 0.028, Cohen's d = 0.33, CTD: 75%FAT nTMS assisted anatomical fibertracking, p = 0.007, Cohen's d = 0.51, 50%FAT nTMS assisted anatomical fibertracking p = 0.015, Cohen's d = 0.46). All other comparisons were non-significant. Distances were measured in millimeters.
3.4. Extent of resection
Median residual tumor volume was 0.3 ± 3.0 cm³ for the whole cohort. Accordingly, the overall EOR was 97.7 ± 11.6% of the tumor volume. In glioblastoma patients, the median residual tumor volume was 0.2 ± 0.8 cm³ (EOR 94.4 ± 8.5%), whereas the median residual volume in grade 2 and 3 gliomas was 4.3 ± 9.25 cm³ (EOR 84.6 ± 17.5%). A gross total resection was achieved in 10 glioblastoma patients (38.5%) and in 2 (16.7%) of the grade 2 and 3 tumors.
LTD measurements did not correlate with residual tumor volume or volume reduction (all p > 0.2). CTDs correlated with residual tumor volume (rs = 0.42, p = 0.015 at 75% FAT). Residual tumor volume was not associated with postoperative motor decline (p = 0.89) as well as with the resection of nTMS spots (p = 0.097) or intersection with the CST on postoperative imaging (p = 0.4). There were no surgery-related complications apart from one hemorrhage which required re-craniotomy and evacuation of the hematoma.
3.5. Subgroup glioblastoma patients
This subgroup comprised 26 patients of which 12 (46.2%) had a preoperative motor deficit. Eight (30.8%) patients developed new motor deficits postoperatively of which 2 (7.7%) were transient. Cortical motor eloquent spots were resected in 6 surgeries (23.1%) and injury of the CST was seen in 9 (34.6%) postoperative images. Persistent decline in motor function was associated with resection of nTMS spots (p = 0.004, Cramer's V = 0.63) and intersection with the CST (p = 0.014, Cramer's V = 0.57). Correspondingly, the positive predictive value for persistent motor deficits after resection of nTMS positive spots was 66.7% and the negative predictive value was 90%. For intersection of CST and resection cavity, the positive predictive value was 83.3% and the negative predictive value was 80%. Closer CTDs were associated with persistent worsening in motor function (p = 0.043, Cohen's d = 0.49 for 75%, p = 0.065, Cohen's d = 0.43 for 50%FAT, in the nTMS assisted anatomical fibertracking, all p > 0.13 for the nTMS based fibertracking).
4. Discussion
This study strictly supports the significance of functional imaging in glioma surgery. Using a nTMS and fibetracking approach, it was possible to resect highly motor eloquent intrinsic brain tumors with a minimal rate of surgery-related permanent motor deficits. Firstly, as a proof-of-principle, the resection of motor eloquent cortical tissue resulted in permanent motor deficits. Secondly, we demonstrated a clear relation between permanent motor deficits and the intraoperative distance to subcortical motor pathways.
In neuro-oncologic neurosurgery, the extent of resection is one main prognostic factor. Furthermore, the surgical goal should be – whenever feasible - a gross total resection in order to significantly improve the prognosis of the patients [[1], [2], [3], [4],8]. Subsequently, it must not be negotiated that both quality of life and long overall survival can only be maintained if patients do not suffer from postoperative motor deficits [7]. The extent of resection in our cohort was within the range of other published cohorts from different centers indicating similar approaches and patient selection [13,[28], [29], [30], [31]].
We observed a significant correlation between the estimated intraoperative distance to the pyramidal tract and the amount of tumor volume reduction as a sign of a more aggressive surgical strategy in patients with lower risk of postoperative unfavorable functional outcome. Yet, the extent of resection and postoperative motor status were independent in our analysis. This finding is congruent with a previously published cohort [28]. In previously published literature, the prevalence of postoperative deficits ranged between 9.75% [38] and 22% [[28], [29], [30],32]. The LTD cut-offs varied between 8 and 12 mm [[28], [29], [30],32]. A second important risk-factor for long-term motor deficits is direct infiltration of the nTMS positive area [[28], [29], [30]].
In our cohort, only 18.4% suffered from a new surgery-induced motor deficit. It needs to be pointed out, that only 9.1% of the patients without a preoperative deficit deteriorated postoperatively. Infiltration of nTMS positive cortex was linked to motor deficits, but did not solely predict motor outcome [[29], [30], [31],33,34]. Moser et al. [33] reported 62% permanent deficits after resection of nTMS positive points and nTMS positive sites outside the precentral gyrus were moreover associated with development of motor deficits. In line with these results, Muir et el [34]. experienced motor decline in 50% of the patients if nTMS spots underwent resection during tumor removal. Additionally, nTMS based tractography could precisely predict motor deficits in their cohort [34]. The spatial relation of motor positive cortical areas and the tumorous lesion suggested a different importance of nTMS positive spots outside the precentral gyrus. No patient with nTMS positive spots at the tumor margin within the postcentral gyrus developed a permanent deficit in our cohort and only two patients with infiltration of supplementary motor areas suffered from a permanent surgery-related deficit. The positive predictive value of nTMS positive cortex at the tumor-brain-interface for functional outcome was rather low in our cohort. The negative predictive values for preserving motor function if eloquent tissue was preserved were remarkably high. These results do not advocate a less aggressive surgical strategy concerning extent of resection. On the contrary, surgery aiming at gross total resection should be performed if motor eloquent tissue is not endangered according to nTMS.
Motor mapping of patients with preoperative motor deficits was possible in our cohort and functional imaging data should be considered in the preoperative planning process of those patients.
The results of our study and previously published data strongly support the prognostic value of presurgical nTMS motor mapping. Furthermore, overlay of nTMS spots and CST-reconstructions with postoperative MRI correlated with postoperative outcome. In cases with infiltrated motor positive area, careful planning of the extent of resection is required in order to preserve the preoperative clinical status of the patients.
4.1. Limitations of the study
Limitations arise from the lack of intraoperative verification of the preoperative planning via electrophysiological monitoring and commonly known limitations of DTI [15,39,40].
Nevertheless, the preoperative nTMS-workflow was highly standardized and nTMS mapping could be conducted in all patients, even in those with severe preoperative deficits. Furthermore, it is broadly accepted that the spatial accuracy of nTMS is comparable to intraoperative mapping [18,19,22]. We have to state that the small sample size might underestimate non-significant results and some results might be underpowered, such as the preoperative tractography. On the contrary, most of our findings are supported by the existing literature. We have to acknowledge that motor deficits were not quantified in a standardized way, however, no patient in the cohort developed a surgery-related plegia or functionally severe impairment.
Moreover, the detailed analysis of the postoperative imaging classified all but one permanent surgery-related deficits correctly. This underlines the significance of functional imaging in surgery of motor eloquent gliomas.
5. Conclusion
In the presented study, postoperative motor function was both associated with the resection of nTMS motor positive spots and with the distance between the resection cavity and the pyramidal tract. nTMS positive spots within the tumor or at the tumor-brain-interface were at risk for being resected during surgery with a subsequent elevated risk for permanent motor deficits. Our data strictly support aggressive surgical strategies in patients without nTMS positive spots at the tumor margin underlined by a negative predictive value of 90% for stable motor function if the motor cortex can be preserved during tumor resection.
5.1. Compliance with Ethical standards
The study design was approved by the Institutional Review Board of Paracelsus Medical University Nuremberg (Registration numbers IRB-2020-022 and IRB-2022-012). All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments. Informed consent for study participation was waived with due to the retrospective study design and statistical analysis of anonymized data.
Disclosures and conflict of interest
T.E. received research grants from „Verein zur Förderung des Tumorzentrums der Universität Erlangen-Nürnberg e.V“
Funding
There was no external funding
Data availability
The anonymous datasets are available from the first author upon reasonable request.
CRediT authorship contribution statement
Thomas Eibl: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Michael Schrey: Writing – review & editing, Supervision, Investigation, Conceptualization. Adrian Liebert: Writing – review & editing, Writing – original draft, Conceptualization. Leonard Ritter: Writing – review & editing, Writing – original draft, Conceptualization. Rüdiger Lange: Supervision, Methodology, Conceptualization. Hans-Herbert Steiner: Supervision. Karl-Michael Schebesch: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Thomas Eibl reports financial support was provided by Verein zur Förderung des Tumorzentrums der Universität Erlangen-Nürnberg e.V. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Molinaro A.M., Hervey-Jumper S., Morshed R.A., Young J., Han S.J., Chunduru P., Zhang Y., Phillips J.J., Shai A., Lafontaine M., et al. Association of maximal extent of resection of contrast-Enhanced and non-contrast-Enhanced tumor with survival within Molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020;6:495–503. doi: 10.1001/jamaoncol.2019.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang J., Lv X., Lu C., Ye X., Chen X., Fu J., Luo C., Zhao Y. Prognostic factors of patients with Gliomas - an analysis on 335 patients with Glioblastoma and other forms of Gliomas. BMC Cancer. 2020;20:35. doi: 10.1186/s12885-019-6511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marko N.F., Weil R.J., Schroeder J.L., Lang F.F., Suki D., Sawaya R.E. Extent of resection of glioblastoma Revisited: Personalized survival Modeling Facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J. Clin. Oncol. 2014;32:774–782. doi: 10.1200/jco.2013.51.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown T.J., Brennan M.C., Li M., Church E.W., Brandmeir N.J., Rakszawski K.L., Patel A.S., Rizk E.B., Suki D., Sawaya R., Glantz M. Association of the extent of resection with survival in glioblastoma: a Systematic review and meta-analysis. JAMA Oncol. 2016;2:1460–1469. doi: 10.1001/jamaoncol.2016.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsigiannis S., Grau S., Krischek B., Er K., Pintea B., Goldbrunner R., Stavrinou P. MGMT-positive vs MGMT-negative patients with glioblastoma: Identification of prognostic factors and resection threshold. Neurosurgery. 2021;88:E323–e329. doi: 10.1093/neuros/nyaa562. [DOI] [PubMed] [Google Scholar]
- 6.McGirt M.J., Chaichana K.L., Gathinji M., Attenello F.J., Than K., Olivi A., Weingart J.D., Brem H., Quiñones-Hinojosa A.r. Independent association of extent of resection with survival in patients with malignant brain astrocytoma: clinical article. Journal of Neurosurgery JNS. 2009;110:156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 7.McGirt M.J., Mukherjee D., Chaichana K.L., Than K.D., Weingart J.D., Quinones-Hinojosa A. Association OF SURGICALLY ACQUIRED motor and LANGUAGE deficits ON overall survival after RESECTION OF glioblastoma MULTIFORME. Neurosurgery. 2009;65:463–470. doi: 10.1227/01.Neu.0000349763.42238.E9. [DOI] [PubMed] [Google Scholar]
- 8.Oppenlander M.E., Wolf A.B., Snyder L.A., Bina R., Wilson J.R., Coons S.W., Ashby L.S., Brachman D., Nakaji P., Porter R.W., et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J. Neurosurg. 2014;120:846–853. doi: 10.3171/2013.12.Jns13184. [DOI] [PubMed] [Google Scholar]
- 9.Pessina F., Navarria P., Cozzi L., Ascolese A.M., Simonelli M., Santoro A., Clerici E., Rossi M., Scorsetti M., Bello L. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? A single institution retrospective experience. J. Neuro Oncol. 2017;135:129–139. doi: 10.1007/s11060-017-2559-9. [DOI] [PubMed] [Google Scholar]
- 10.Sanai N., Polley M.Y., McDermott M.W., Parsa A.T., Berger M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 11.De Witt Hamer P.C., Robles S.G., Zwinderman A.H., Duffau H., Berger M.S. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J. Clin. Oncol. 2012;30:2559–2565. doi: 10.1200/jco.2011.38.4818. [DOI] [PubMed] [Google Scholar]
- 12.Verburg N., de Witt Hamer P.C. State-of-the-art imaging for glioma surgery. Neurosurg. Rev. 2021;44:1331–1343. doi: 10.1007/s10143-020-01337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss Lucas C., Faymonville A.M., Loução R., Schroeter C., Nettekoven C., Oros-Peusquens A.M., Langen K.J., Shah N.J., Stoffels G., Neuschmelting V., et al. Surgery of motor eloquent glioblastoma guided by TMS-Informed tractography: Driving resection Completeness towards Prolonged survival. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.874631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss Lucas C., Tursunova I., Neuschmelting V., Nettekoven C., Oros-Peusquens A.M., Stoffels G., Faymonville A.M., Jon S.N., Langen K.J., Lockau H., et al. Functional MRI vs. navigated TMS to optimize M1 seed volume delineation for DTI tractography. A prospective study in patients with brain tumours adjacent to the corticospinal tract. Neuroimage Clin. 2017;13:297–309. doi: 10.1016/j.nicl.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson F., Abdullah K.G., Verma R., Brem S. Tractography and the connectome in neurosurgical treatment of gliomas: the premise, the progress, and the potential. Neurosurg. Focus. 2020;48:E6. doi: 10.3171/2019.11.FOCUS19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieg S.M., Shiban E., Buchmann N., Gempt J., Foerschler A., Meyer B., Ringel F. Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J. Neurosurg. 2012;116:994–1001. doi: 10.3171/2011.12.Jns111524. [DOI] [PubMed] [Google Scholar]
- 17.Krieg S.M., Shiban E., Buchmann N., Meyer B., Ringel F. Presurgical navigated transcranial magnetic brain stimulation for recurrent gliomas in motor eloquent areas. Clin. Neurophysiol. 2013;124:522–527. doi: 10.1016/j.clinph.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Weiss Lucas C., Nettekoven C., Neuschmelting V., Oros-Peusquens A.M., Stoffels G., Viswanathan S., Rehme A.K., Faymonville A.M., Shah N.J., Langen K.J., et al. Invasive versus non-invasive mapping of the motor cortex. Hum. Brain Mapp. 2020;41:3970–3983. doi: 10.1002/hbm.25101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forster M.T., Hattingen E., Senft C., Gasser T., Seifert V., Szelenyi A. Navigated transcranial magnetic stimulation and functional magnetic resonance imaging: advanced adjuncts in preoperative planning for central region tumors. Neurosurgery. 2011;68:1317–1324. doi: 10.1227/NEU.0b013e31820b528c. ; discussion 1324-1315. 10.1227/NEU.0b013e31820b528c. [DOI] [PubMed] [Google Scholar]
- 20.Picht T., Frey D., Thieme S., Kliesch S., Vajkoczy P. Presurgical navigated TMS motor cortex mapping improves outcome in glioblastoma surgery: a controlled observational study. J. Neuro Oncol. 2016;126:535–543. doi: 10.1007/s11060-015-1993-9. [DOI] [PubMed] [Google Scholar]
- 21.Picht T., Mularski S., Kuehn B., Vajkoczy P., Kombos T., Suess O. Navigated transcranial magnetic stimulation for preoperative functional diagnostics in brain tumor surgery. Neurosurgery. 2009;65:93–98. doi: 10.1227/01.NEU.0000348009.22750.59. discussion 98-99. 10.1227/01.Neu.0000348009.22750.59. [DOI] [PubMed] [Google Scholar]
- 22.Picht T., Schmidt S., Brandt S., Frey D., Hannula H., Neuvonen T., Karhu J., Vajkoczy P., Suess O. Preoperative functional mapping for rolandic brain tumor surgery: comparison of navigated transcranial magnetic stimulation to direct cortical stimulation. Neurosurgery. 2011;69:581–588. doi: 10.1227/NEU.0b013e3182181b89. discussion 588. 10.1227/NEU.0b013e3182181b89. [DOI] [PubMed] [Google Scholar]
- 23.Picht T., Strack V., Schulz J., Zdunczyk A., Frey D., Schmidt S., Vajkoczy P. Assessing the functional status of the motor system in brain tumor patients using transcranial magnetic stimulation. Acta Neurochir. 2012;154:2075–2081. doi: 10.1007/s00701-012-1494-y. [DOI] [PubMed] [Google Scholar]
- 24.Frey D., Schilt S., Strack V., Zdunczyk A., Rosler J., Niraula B., Vajkoczy P., Picht T. Navigated transcranial magnetic stimulation improves the treatment outcome in patients with brain tumors in motor eloquent locations. Neuro Oncol. 2014;16:1365–1372. doi: 10.1093/neuonc/nou110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conti A., Raffa G., Granata F., Rizzo V., Germano A., Tomasello F. Navigated transcranial magnetic stimulation for "somatotopic" tractography of the corticospinal tract. Neurosurgery. 2014;10(Suppl 4):542–554. doi: 10.1227/NEU.0000000000000502. discussion 554. 10.1227/NEU.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 26.Frey D., Strack V., Wiener E., Jussen D., Vajkoczy P., Picht T. A new approach for corticospinal tract reconstruction based on navigated transcranial stimulation and standardized fractional anisotropy values. Neuroimage. 2012;62:1600–1609. doi: 10.1016/j.neuroimage.2012.05.059. [DOI] [PubMed] [Google Scholar]
- 27.Krieg S.M., Buchmann N.H., Gempt J., Shiban E., Meyer B., Ringel F. Diffusion tensor imaging fiber tracking using navigated brain stimulation--a feasibility study. Acta Neurochir. 2012;154:555–563. doi: 10.1007/s00701-011-1255-3. [DOI] [PubMed] [Google Scholar]
- 28.Belotti F., Tuncer M.S., Rosenstock T., Ivren M., Vajkoczy P., Picht T. Predicting the extent of resection of motor-eloquent gliomas based on TMS-guided fiber tracking. Brain Sci. 2021;11 doi: 10.3390/brainsci11111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenstock T., Grittner U., Acker G., Schwarzer V., Kulchytska N., Vajkoczy P., Picht T. Risk stratification in motor area-related glioma surgery based on navigated transcranial magnetic stimulation data. J. Neurosurg. 2017;126:1227–1237. doi: 10.3171/2016.4.JNS152896. [DOI] [PubMed] [Google Scholar]
- 30.Rosenstock T., Häni L., Grittner U., Schlinkmann N., Ivren M., Schneider H., Raabe A., Vajkoczy P., Seidel K., Picht T. Bicentric validation of the navigated transcranial magnetic stimulation motor risk stratification model. J. Neurosurg. 2021:1–13. doi: 10.3171/2021.3.Jns2138. [DOI] [PubMed] [Google Scholar]
- 31.Rosenstock T., Tuncer M.S., Münch M.R., Vajkoczy P., Picht T., Faust K. Preoperative nTMS and intraoperative Neurophysiology - a Comparative analysis in patients with motor-eloquent glioma. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.676626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sollmann N., Wildschuetz N., Kelm A., Conway N., Moser T., Bulubas L., Kirschke J.S., Meyer B., Krieg S.M. Associations between clinical outcome and navigated transcranial magnetic stimulation characteristics in patients with motor-eloquent brain lesions: a combined navigated transcranial magnetic stimulation-diffusion tensor imaging fiber tracking approach. J. Neurosurg. 2018;128:800–810. doi: 10.3171/2016.11.Jns162322. [DOI] [PubMed] [Google Scholar]
- 33.Moser T., Bulubas L., Sabih J., Conway N., Wildschutz N., Sollmann N., Meyer B., Ringel F., Krieg S.M. Resection of navigated transcranial magnetic stimulation-positive Prerolandic motor areas Causes permanent impairment of motor function. Neurosurgery. 2017;81:99–110. doi: 10.1093/neuros/nyw169. [DOI] [PubMed] [Google Scholar]
- 34.Muir M., Prinsloo S., Michener H., Traylor J.I., Patel R., Gadot R., de Almeida Bastos D.C., Kumar V.A., Ferguson S., Prabhu S.S. TMS seeded diffusion tensor imaging tractography predicts permanent neurological deficits. Cancers. 2022;14 doi: 10.3390/cancers14020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farquharson S., Tournier J.D., Calamante F., Fabinyi G., Schneider-Kolsky M., Jackson G.D., Connelly A. White matter fiber tractography: why we need to move beyond DTI. J. Neurosurg. 2013;118:1367–1377. doi: 10.3171/2013.2.JNS121294. [DOI] [PubMed] [Google Scholar]
- 36.Eibl T., Schrey M., Weigel J., Liebert A., Lange R., Städt M., Eff F., Holtmannspötter M., Steiner H.H. Assessing the feasibility of mapping the tibialis anterior muscle with navigated transcranial magnetic stimulation in neuro-oncologic patients. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-23444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krieg S.M., Lioumis P., Makela J.P., Wilenius J., Karhu J., Hannula H., Savolainen P., Lucas C.W., Seidel K., Laakso A., et al. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir. 2017;159:1187–1195. doi: 10.1007/s00701-017-3187-z. [DOI] [PubMed] [Google Scholar]
- 38.Raffa G., Scibilia A., Conti A., Cardali S.M., Rizzo V., Terranova C., Quattropani M.C., Marzano G., Ricciardo G., Vinci S.L., Germanò A. Multimodal surgical treatment of high-grade gliomas in the motor area: the Impact of the combination of navigated transcranial magnetic stimulation and Fluorescein-guided resection. World Neurosurg. 2019;128:e378–e390. doi: 10.1016/j.wneu.2019.04.158. [DOI] [PubMed] [Google Scholar]
- 39.Yen P.S., Teo B.T., Chiu C.H., Chen S.C., Chiu T.L., Su C.F. White matter tract involvement in brain tumors: a diffusion tensor imaging analysis. Surg. Neurol. 2009;72:464–469. doi: 10.1016/j.surneu.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Henderson F., Jr., Parker D., Vijayakumari A.A., Elliott M., Lucas T., McGarvey M.L., Karpf L., Desiderio L., Harsch J., Levy S., et al. Enhanced fiber tractography using Edema correction: Application and evaluation in high-grade gliomas. Neurosurgery. 2021;89:246–256. doi: 10.1093/neuros/nyab129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymous datasets are available from the first author upon reasonable request.