Abstract
The metabolic disorders caused by diabetes can lead to various complications, including male spermatogenesis dysfunction. Exploring effective therapeutics that attenuate diabetes mellitus (DM)-induced male subfertility is of great importance. Pharmaceuticals targeting PPARα activation such as bezafibrate have been regarded as an important strategy for patients with diabetes. In this study, we use streptozocin (STZ) injection to establish a type 1 DM mice model and use bezafibrate to treat DM mice and evaluate the effects of bezafibrate on the spermatogenic function of the DM male mice. Bezafibrate treatment exhibited protective effects on DM-induced spermatogenesis deficiency, as reflected by increased testis weight, improved histological morphology of testis, elevated sperm parameters, increased serum testosterone concentration as well as increased mRNA levels of steroidogenesis enzymes. Meanwhile, testicular cell apoptosis, inflammation accumulation and oxidative stress status were also shown to be alleviated by bezafibrate compared with the DM group. In vivo and in vitro studies, PPARα specific inhibitor and PPARα knockout mice were further used to investigate the role of PPARα in the protective effects of bezafibrate on DM-induced spermatogenesis dysfunction. Our results indicated that the protection of bezafibrate on DM-induced spermatogenesis deficiency was abrogated by PPARα inhibition or deletion. Our study suggested that bezafibrate administration could ameliorate DM-induced spermatogenesis dysfunction and may represent a novel practical strategy for male infertility.
Keywords: Bezafibrate, Diabetes mellitus (DM), Spermatogenesis dysfunction, PPARα, Inflammation, Oxidative stress
1. Introduction
Diabetes mellitus (DM) is one of the major health problems worldwide and the prevalence of diabetes is projected to increase to 4.4% globally by 2030 [1]. Persistent hyperglycemia contributes to a series of diabetic complications including diabetic cardiomyopathy, diabetic nephropathy, diabetic retinopathy as well as reproductive disorders [[2], [3], [4], [5], [6], [7], [8]]. Recently, accumulating evidence indicates that DM-induced hyperglycemia impairs spermatogenesis and male reproductive function [9,10]. Despite the fact that the crucial role of diabetes in male spermatogenesis dysfunction has been noticed, the molecular mechanism underlying diabetes-induced male subfertility remains unclear. More and more evidence suggested that endocrine disorders such as disruption in the hypothalamic-pituitary gonadal axis, the direct and indirect effects of insulin on the testis and spermatozoa and disturbances in leptin signaling contributed to male spermatogenesis dysfunction of DM mice [11,12]. In addition, increased oxidative stress, inflammation and cell apoptosis as well as abnormal zinc metabolism also participated in the male spermatogenesis deficiency induced by DM [[13], [14], [15], [16]].
Peroxisome proliferator activated receptor alpha (PPARα) is a member of the ligand-regulated nuclear receptors family. PPARα has been demonstrated to participate in the regulation of insulin sensitivity [17]. PPARα activation could improve inflammation in pre-clinical models of non-alcoholic fatty liver disease [18]. Recently, it has been reported that a PPARα agonist attenuated hyperglycemia-induced oxidative stress in pancreatic cells [19]. Moreover, pharmaceuticals targeting PPARα activation have been regarded as an important strategy for patients with diabetes, metabolic syndrome, and cardiovascular diseases [[20], [21], [22]]. Bezafibrate, a PPARα agonist, has been confirmed to ameliorate glucotoxicity and reduce insulin resistance in STZ mice [23]. Moreover, bezafibrate could ameliorate arterial stiffness in hypertriglyceridemic patients with type 2 diabetes mellitus [24]. However, there is no evidence about the protective role of bezafibrate on diabetes-induced male spermatogenesis dysfunction. STZ injection-induced DM mice model can mimic the clinical features of patients with type 1 diabetes and has been used to employ diabetes-related male fertility in several previous studies [[25], [26], [27]]. Thus, this study was designed to investigate the effects of bezafibrate on testicular function using streptozotocin (STZ)-induced type 1 DM mice model and to discover the underlying molecular mechanism.
2. Materials and methods
2.1. Animals
8–10 weeks old male C57BL/6J mice weighing between 20 and 26 g were obtained from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (Beijing, China). The mice were kept in the experimental animal center of Renmin Hospital of Wuhan University where the temperature was maintained between 20 °C and 25 °C, relative humidity near 50% and a 12-h light/dark cycle. The animal experiments included in our study were performed according to the Guidelines for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication, revised 2011), the Guidelines for the Care and Use of Laboratory Animals of the Chinese Animal Welfare Committee and were ratified by the Animal Use Committees of Renmin Hospital of Wuhan University and Ethical Guidelines for Publication of Research Results in Biology of Reproduction.
The mice were unbiasedly apportioned into four groups (10 mice per group): Control (Con) + vehicle, Con + bezafibrate, DM + vehicle, DM + bezafibrate. To establish the diabetes model, mice were intraperitoneally injected with fresh 60 mg/kg STZ, which was dissolved into 0.1 M sodium citrate buffer, on 5 consecutive days [28]. Mice with fasting blood glucose >13.9 mmol/L in three independent measurements were defined as having diabetes and were used for further studies [29]. 8 weeks after the mice were diagnosed with diabetes, 100 mg/kg bezafibrate (sc-204650C, Santa Cruz Biotechnology, CA, USA) or vehicle (1% methylcellulose) was orally administered to these mice once per day at 10:00 a.m. for 4 weeks [30].
To further evaluate the role of PPARα in the effects of bezafibrate on DM-related spermatogenesis function, PPARα global knockout mice (Pparatm1Gonz, Strain #:008154), which were obtained from Jackson Laboratory, were also used in this study. Genotyping was performed with the specific primers (common primer: 5′-GAGAAGTTGCAGGAGGGGATTGTG-3'; wild type reverse primer: 5′-CCCATTTCGGTAGCAGGTAGTCTT-3'; mutant reverse: 5′-GCAATCCATCTTGTTCAATGGC-3′). This mouse line was maintained on a C57BL/6J background, and the homozygous mice were viable and fertile. The PPARα knockout mice were aged 8-10 weeks and weighed between 20 and 26 g. These mice were also subjected to STZ injection and bezafibrate administration as described above. Fresh STZ was intraperitoneally injected in adult male mice at a dose of 60 mg/kg to establish a diabetes model for 5 consecutive days. 8 weeks after the mice were diagnosed with diabetes, 100 mg/kg bezafibrate or vehicle (1% methylcellulose) was orally administered to these mice once per day at 10:00 a.m. for 4 weeks.
2.2. Blood and organ collection
Four weeks after bezafibrate treatment, all mice were sacrificed with an overdose of pentobarbital sodium (200 mg/kg), and the body weight as well as tibia length were measured and recorded. Blood was obtained from the carotid artery and centrifuged at 1000 g for 15 min at 4 °C and the serum was separated for the detection of testosterone and adipokines levels. The testis and epididymis were immediately removed, washed with phosphate buffer and the weight of the testis was recorded. A part of the testis was preserved in Bouin's solution for histopathology, while the remaining part of the testis was frozen at −80 °C for further detection.
Blood triglyceride (TG), nonesterified fatty acid (NEFA), glycosylated hemoglobin A1c (HbA1c) and insulin levels detection.
Plasma TG (#E-BC-K261-M) and NEFA (#E-BC-K013-S) levels were determined by commercial assaying kits according to the manufacturer's instructions. The kits were purchased from Elabscience Biotechnology Co., Ltd. (Wuhan, China). Blood HbA1c levels were measured using a mouse HbA1c ELISA kit (#EKF57810) from Biomatik (Ottawa, Canada). Insulin levels were determined by a mouse insulin ELISA kit (#EKL54733) from Biomatik.
3. Adipokines detection in serum and testis
The testis tissues were cut into smaller pieces and homogenized in phosphate buffered saline, followed by centrifugation at 1000g for 10 min at 4 °C. Serum and testis levels of leptin, adiponectin, irisin and C1q/tumor necrosis factor-related protein-3 (CTRP3) were determined according to the manufacturer's instructions. Mouse leptin ELISA kit (#ADI-900-019A) was provided by Enzo Life Sciences (Raamsdonksveer, The Netherlands). Mouse adiponectin ELISA kit (#EKL54022) and mouse irisin ELISA kit were obtained from Biomatik. The CTRP3 detection kit (#580200) was purchased from Cayman Chemical (Ann Arbor, MI, USA).
3.1. Histological analysis and TUNEL staining
Testis tissue samples were fixed in Bouin's solution, dehydrated in ethanol, and embedded in paraffin according to our previous study [31]. Sections were cut into 5 μm thickness with a microtome and stained with hematoxylin and eosin (H&E) to detect morphology. The histological assessment was performed by two researchers blindly using a light microscope. For each mouse, 20 round seminiferous tubules were randomly selected, and their diameters were measured at × 100 magnification using Image-Pro Plus 6.0.
Briefly, paraformaldehyde fixed, paraffin embedded testis sections were detected by the transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) according to the manufacturer's instructions using a commercially available kit (Millipore, USA). Finally, the sections were observed and captured under a fluorescence microscope (BX51, Olympus, Japan). A total of 25 fields were randomly selected in each group (5 fields/mice) and 100 cells were counted in each field. The results were expressed as percentages (%).
3.2. DNA damage analysis
The testis tissues were cut into smaller pieces and homogenized in 10% phosphate buffered saline, followed by centrifugation at 1000g for 10 min at 4 °C. Testicular 8-hydroxydeoxyguanosine (8-OHdG) level was used to determine DNA damage and the level of 8-OHdG in testis tissues was measured by a commercial QuickDetect™ 8-OHdG (Mouse) ELISA kit (#E4441-100) regarding the manufacturer's procedures. The assaying kit was obtained from BioVision (Milpitas, CA, USA).
3.3. Real-Time polymerase chain reaction analysis
Total RNA was extracted from frozen testis tissues via TRIzol reagent. The mRNA was subsequently reversely transcribed to cDNA using a Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). Gene expression was analyzed with Real-Time PCR using LightCycler 480 SYBR Green 1 Master Mix (Roche). The primers were selected from GenBank and synthesized by Takara Biomedical Technology (Beijing, China). All samples were analyzed in triplicate and results were normalized to GAPDH as the housekeeping gene. Primer sequences are listed in Supplemental Table 1.
3.4. Protein extraction and Western blot assay
RIPA buffer was used to extract the protein from the testis according to previous studies [31,32]. Lysates were centrifuged at 4 °C for 10 min. Protein concentration in supernatants was measured using an Extra Sense BCA Protein Assay Kit (K814-2500, Biovision) according to the manufacturer's instructions. Sample with equal amounts of proteins (20 μg) were separated by 10% SDS-PAGE gels and transferred to PVDF membranes. Membranes were blocked with 5% nonfat milk in Tris-buffer containing Tween 20 (0.1%) for 1 h, and subsequently incubated overnight at 4 °C with the following primary antibodies: rabbit anti-Bax antibody (1:500 dilution, #ab182733, Abcam, Cambridge, MA, USA), rabbit anti-Bcl-2 antibody (#ab182858, Abcam, 1:1000 dilution), rabbit anti-phosphor-p65 antibody (ab183559, Abcam, 1:1000 dilution), rabbit anti-p65 antibody (ab32536, Abcam, 1:1000 dilution), rabbit anti-nucleotide-binding oligomerization domain-like receptor with a pyrin domain 3 (NLRP3) antibody (ab270449, Abcam, 1:500 dilution), rabbit anti-PPARα antibody (ab61182, Abcam, 1:500 dilution), rabbit anti-PPARβ antibody (1:300 dilution, #sc-74517, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-PPARγ antibody (ab209350, Abcam, 1:500 dilution), rabbit anti-phosphor-protein kinase B (AKT) antibody (ab38449, Abcam, 1:500 dilution), rabbit anti-AKT antibody (ab8805, Abcam, 1:500 dilution), rabbit anti-phosphor-AMP-activated protein kinase α (AMPKα) antibody (1:1000 dilution, #2535, Cell Signaling Technology, Danvers, MA,USA), rabbit anti-AMPKα antibody (#2603, Cell Signaling Technology, 1:1000 dilution, #2535), rabbit anti-GAPDH antibody (ab8254, Abcam, 1:1000 dilution). Membranes were then probed with secondary antibodies at room temperature for 1 h. The density of specific bands was measured using ECL (Bio-Rad, USA) and normalized to GAPDH and finally calculated as fold change to the matched group.
3.5. Spermatological parameters
The caudal epididymis of the mice was dissected away from the fat and immediately placed into Ringer's solution and chopped into small pieces to mobilize the spermatozoa. The sperm count, sperm viability and sperm motility were determined according to previously described protocols [[31], [32], [33]]. In brief, the epididymis was cut to obtain the sperm. After that, the sperm was released onto a sterile clean glass slide and further diluted with Ringer's solution and properly mixed. The sperm motility was accessed by two researchers blindly using a light microscope. The viability of the sperm was detected by eosin/nigrosin staining as previously described. For the determination of the sperm count, the sperm suspension obtained from the incised cauda epididymis was diluted with Ringer's solution. A drop of the suspension was charged into the Neubauer hemocytometer chamber and counted under a light microscope by two researchers blindly [34]. Detached heads of sperm cells in each group were calculated by two researchers under a microscope blindly by investigating 200 sperm cells for each slide and the result was expressed as a percentage (%).
Sperm samples, with an adjusted concentration of 10 × 106 spermatozoa/ml, were then incubated at 37 °C and 5% CO2 in PBS supplemented with 0.9 mmol/L CaCl2, 0.5 mmol/L MgCl2, 0.3% (w/v) BSA, 1 mmol/L sodium pyruvate, 10 mmol/L sodium lactate, and 1% penicillin/streptomycin, pH = 7.4 with or without 25 mmol/L d-glucose to study the in vivo hyperglycemia environment on the sperm function with or without bezafibrate treatment (100 μmol/L) [35,36]. To determine the effects of different inhibitors on sperm function, sperm were first isolated from DM mice and PPARα (GW6471, 20 μmol), PPARβ/δ (GSK0660, 1 μmol, sc-203985, Santa Cruz Biotechnology) and PPARγ (GW9662, sc-202641, 10 μmol, Santa Cruz Biotechnology, CA, USA) antagonists were added into the sperm culture medium [37].
3.6. TM3 and TM4 cell culture and treatment
To explore the target cell of bezafibrate on HG-induced impairment of male spermatogenesis, we used TM3 and TM4 cells for further analysis. TM3 mouse Leydig cells were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China). TM4 mouse Sertoli cells were purchased from the American Type Culture Collection (Manassas, USA). The two cell lines share many of the characteristics of primary cells and were used as a surrogate for primary cells. TM3 and TM4 cells were cultured in DMEM/F12 medium supplemented with 10% FBS for 48 h, after which the cells were cocultured with or without 25 mmol/L d-glucose to mimic the in vivo hyperglycemia environment of diabetes with or without bezafibrate treatment (100 μmol/L). To further determine the effects of PPARα on Sertoli cell and Leydig cell function, PPARα antagonist (GW6471, 20 μmol) was added into the culture medium.
3.7. Sertoli cell viability detection
Cell viability of TM4 mouse Sertoli cells was detected by cell counting kits (CCK-8, Dojindo Molecular Technologies, Rockville, MD, USA) according to the manufacturer's protocol [32].
3.8. Testosterone level assessment
Testosterone was measured using a testosterone (mouse/rat) ELISA Kit from Biovision (#K7418-100). The detecting process was carried out according to the manufacturer's instructions as previously described [38,39].
3.9. Assessment of the fertility of male mice
The mice receiving different treatments were used in a breeding assay. Each male mouse (n = 6) was caged with two females for five days. Consequently, a total of 60 females were involved in the study, and their vaginal plugs were checked every morning. In each litter, the birth date and pups’ number were recorded and undergone statistics.
3.10. Detection of testicular caspase 3 activity and caspase 1 activity
The excised testis tissues were cut into smaller pieces and homogenized in 10% phosphate buffered saline, followed by centrifugation for 10 min at 4 °C. Caspase 3 activity was determined using the Colorimetric Assay Kit (E-CK-A311) from Elabscience Biotechnology Co., Ltd. (Wuhan, China) according to the manufacturer's instructions. The caspase 1 activity in the testis was measured using a Caspase 1 Colorimetric Assay Kit (#K111, BioVision, Mountain View, USA).
3.11. Testicular oxidative stress status
Reactive oxygen species (ROS) production in the testis was determined using electron spin resonance (ESR) spectroscopy (Bruker, Karlsruhe, Germany) with 5,5-dimetyl-1-pyrroline N-oxide (DMPO, Sigma) at a final concentration of 1 mol/L as described previously [40]. Testicular antioxidant/oxidative stress markers were detected using the testicular supernatants following well-established methods. Firstly, fresh testis tissues were cut into smaller pieces and homogenized in 10% phosphate buffered saline, followed by centrifugation at 1000g for 10 min at 4 °C. The supernatant was obtained from the testis homogenate and further used for the determination of the concentrations of malondialdehyde (MDA), catalase (CAT), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) using commercial assay kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The protein carbonyl content was determined by the Protein Carbonyl Assay Kit (#ab126287, Abcam, Cambridge, MA, USA) using DNPH, according to a previous study [41].
3.12. Determination of inflammatory parameters in mice testis
The testis tissues were cut into smaller pieces and homogenized in 10% phosphate buffered saline, followed by centrifugation at 1000g for 10 min at 4 °C. Inflammatory parameters including tumor necrosis factor-α (TNF-α), myeloperoxidase (MPO), interleukin (IL-6), IL-1β and IL-18 were detected by commercial assay kits following the instructions included by the manufacturer. TNF-α mouse ELISA kit (#BMS607-3TEN), MPO mouse ELISA kit (#EMMPO), IL-6 mouse ELISA kit (#KMC0061), IL-1β mouse ELISA kit (#BMS6002) and IL-18 mouse ELISA kit (BMS618-3) were purchased from eBioscience (San Diego, CA, USA).
3.13. Statistical analysis
Data obtained from the present study were analyzed using SPSS 22.0 software, and results are presented as mean ± SD. Single comparisons between two groups were performed using the t-test. Multiple comparisons among groups were analyzed by one-way ANOVA followed by the post hoc Tukey test. The percentage of plugs between different groups was compared using a chi-square test. Values of p < 0.05 were considered statistically significant.
4. Results
4.1. Bezafibrate attenuated the metabolic changes of DM mice
To evaluate the effects of bezafibrate on diabetes-induced spermatogenic dysfunction, the metabolic disorders of DM mice were first detected. As indicated in Fig. 1 A, body weight was reduced in the DM group compared with the Con group. After bezafibrate treatment, the body weight was increased. Meanwhile, the plasma TG and NEFA levels were both increased in the DM group and conversely decreased by bezafibrate treatment (Fig. 1B and C). The influence of bezafibrate on serum glucose levels was also determined. As suggested, blood glucose level and percentage of HbA1c were both increased in DM mice (Fig. 1D and E). After bezafibrate intervention, the elevated serum glucose level and HbA1c percentage were slightly reduced (Fig. 1D and E). Blood insulin level was reduced in STZ-induced DM mice, and bezafibrate was shown to have no influence on the dramatically decreased insulin level of DM mice (Fig. 1F).
Fig. 1.
Adipokines variation of DM mice after bezafibrate treatment. (A) The body weight of the mice in the indicated groups (n = 6). (B) Plasma TG levels (n = 6). (C) Plasma NEFA levels (n = 6). (D) Blood glucose levels (n = 6). (E) Percentage of HbA1C (n = 6). (F) Blood insulin levels (n = 6). (G) Plasma and testis leptin levels (n = 6). (H) Plasma and testis adiponectin levels (n = 6). (I) Plasma and testis irisin levels (n = 6). (J) Plasma and testis CTRP3 levels (n = 6). Data are presented as the mean ± SD. For A to D, the statistical analysis was carried out by one-way ANOVA. *P < 0.05 vs the matched control.
As our previous studies as well as other researchers’ studies revealed a crucial role of adipokines on spermatogenesis [[42], [43], [44]], the serum and testicular levels of adipokines including leptin, adiponectin, irisin and CTRP3 were all evaluated (Fig. 1G–J). The data in the present study indicated that concentrations of leptin, adiponectin, irisin and CTRP3 were all decreased in both plasma and testis of DM mice. However, only adiponectin and irisin levels in plasma and testis were improved by bezafibrate (Fig. 1H and I).
4.2. Bezafibrate ameliorated the spermatogenesis deficiency and improved the fertility of DM mice
The effects of bezafibrate on the spermatogenesis function of DM mice were subsequently detected. The results indicated that the testis weight/tibia length was decreased by DM and restored by bezafibrate (Fig. 2A). The histological morphology also revealed that the impaired histological changes as well as reduced diameter of seminiferous tubules were both restored by bezafibrate treatment (Fig. 2B–C and Fig. S1). Sperm parameter including sperm count, sperm viability and sperm motility was decreased in STZ-induced DM mice, and the percentage of detached head of sperms were increased in DM mice (Fig. 2D and E). After bezafibrate treatment, these sperm parameters were all improved (Fig. 2D and E). We also detected the serum testosterone level and the mRNA levels of steroidogenesis enzymes. The results suggested that the serum testosterone concentration as well as mRNA levels of steroidogenesis enzymes including StAR, P450scc, P450c17, 3β-HSD and 17β-HSD were all decreased in the DM group and further increased by bezafibrate treatment (Fig. 2F and G).
Fig. 2.
Inflammatory factors and adipokines variation in DM mice after empagliflozin therapy. (A) Testis weight/tibia length (n = 6). (B) The histological morphology of the indicated groups (n = 6). (C) The diameter of seminiferous tubules of the indicated groups (n = 6). (D–E) Sperm count, sperm viability, sperm motility and percentage of detached head of sperm (n = 6). (F) Serum testosterone concentration (n = 6). (G) mRNA levels of steroidogenesis enzymes (n = 6). For A-G, data are presented as the mean ± SD and the statistical analysis was carried out by one-way ANOVA. *P < 0.05 vs the matched control.
To further investigate the effects of bezafibrate on male fertility, the percentage of plugs and litter size were calculated in this study. The results indicated that the percentage of plugs was decreased in DM male mice compared with the control group (33.33% vs 53.33%, P < 0.05). After bezafibrate treatment, the reduction of plugs was improved (46.67% vs 33.33%, P < 0.05). We also found that the litter size of DM male adult mice was reduced compared with that in the control group (3.86 ± 0.69 pups per litter vs 6.62 ± 0.92 pups per litter, P < 0.05). Moreover, the litter size of DM male adult mice was further increased by bezafibrate treatment (5.38 ± 0.81 pups per litter vs 3.86 ± 0.69 pups per litter, P < 0.05).
4.3. Bezafibrate attenuated cell apoptosis in the testis of DM mice
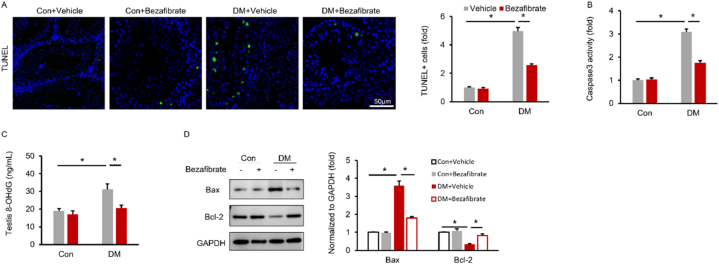
TUNEL staining was used to determine the cell apoptosis of testis. The results demonstrated that the percentage of apoptotic cells was increased in DM mice and decreased after bezafibrate treatment (Fig. 3A). Testicular caspase 3 activity was also measured and the results revealed that bezafibrate suppressed the elevated caspase 3 activity in DM mice (Fig. 3B). DNA damage was increased by DM, as manifested by elevated testicular 8-OHdG concentration. This pathological alteration was attenuated by bezafibrate (Fig. 3C). The inhibitory effects of bezafibrate on cell apoptosis were further confirmed by Western blot results showing that bezafibrate decreased the protein expression of Bax, but increased the protein expression of Bcl-2 in DM mice (Fig. 3D).
Fig. 3.
Bezafibrate attenuates testicular cell apoptosis in DM mice (A) TUNEL staining in the indicated groups (n = 6). (B) The testicular caspase 3 activity of the indicated groups (n = 6). (C) Testis 8-OHdG levels (n = 6). (D) Protein expression of Bax and Bcl-2 of the indicated groups (n = 6). The uncropped blots were listed in Supplementary Fig. 1. Data are presented as the mean ± SD. For A-D, the statistical analysis was carried out by one-way ANOVA. *P < 0.05 vs the matched control.
4.4. Bezafibrate attenuated testicular inflammation status in mice with diabetes
Increased inflammation was regarded as an important factor that participated in the process of DM-induced spermatogenesis dysfunction [45,46]. The testicular inflammation status was subsequently evaluated. The results suggested that the mRNA levels of TNF-α, IL-6, monocyte chemoattractant protein-1 (MCP-1), IL-10, inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) were all increased in DM mice (Fig. 4A and B). After bezafibrate treatment, the increased mRNA levels of TNF-α, IL-6, MCP-1, IL-10, iNOS and COX-2 were all decreased (Fig. 4A and B). Meanwhile, testicular protein concentrations of TNF-α, MPO and IL-6 were also elevated by DM but decreased by bezafibrate (Fig. 4C). NF-κB is crucial for the transcription of several inflammatory factors [47,48], therefore we detected phosphorylation of p65, and found that bezafibrate treatment decreased phosphorylation of p65 in DM mice (Fig. 4D and E). NF-κB activated the NLRP3 signaling pathway, and NLRP3 played a key role in spermatogenesis dysfunction [49,50]. NLRP3 protein expression was increased in the testis of DM mice, and this elevation was suppressed by bezafibrate treatment (Fig. 4D and E). Downstream targets of NLRP3 including IL-1β, IL-18 and caspase 1 activity were also detected. The data demonstrated that IL-1β, IL-18 levels and caspase 1 activity were all increased by STZ-induced DM (Fig. 4F). After bezafibrate treatment, IL-1β, IL-18 levels and caspase 1 activity were all decreased (Fig. 4F).
Fig. 4.
Bezafibrate decreases testicular inflammation accumulation in DM mice (A–B) mRNA levels of TNF-α, IL-6, MCP-1, IL-10, iNOS and COX-2 (n = 6). (C) Testicular concentrations of TNF-α, MPO and IL-6 (n = 6). (D–E) The phosphorylation of p65 and protein expression of NLRP3 (n = 6). The uncropped blots were listed in Supplementary Fig. 1 (F) Testicular IL-1β, IL-18 levels and caspase 1 activity (n = 6). Data are presented as the mean ± SD. For A-F, the statistical analysis was carried out by one-way ANOVA. *P < 0.05 vs the matched control.
4.5. Bezafibrate attenuated oxidative stress status in mice with diabetes
As bezafibrate was an agonist for PPARs, mRNA levels of PPARα, PPARβ and PPARγ were detected after bezafibrate treatment. As revealed, only mRNA and protein expression of PPARα were slightly increased after bezafibrate administration (Fig. 5A and B). PPARβ and PPARγ were not changed after bezafibrate treatment (Fig. 5A and B). PPARα mRNA and protein expression were not altered in DM + vehicle mice but largely increased in DM mice with bezafibrate administration. Akt and AMPKα were the downstream targets of PPARα, therefore the phosphorylation of Akt and AMPKα was detected. The results revealed that the phosphorylation of Akt and AMPKα were downregulated in the DM group but upregulated by bezafibrate treatment (Fig. 5C).
Fig. 5.
Fatty acid oxidation was enhanced by bezafibrate in DM mice. (A–B) mRNA levels and protein expression of PPARα, PPARβ and PPARγ (n = 6). The uncropped blots were listed in Supplementary Fig. 1 (C) The phosphorylation of AKT and AMPKα (n = 6). The uncropped blots were listed in Supplementary Fig. 1 (D) mRNA levels of ACOX1, CPT1A and CD36 (n = 6). (E) mRNA levels of PGC1-α, NRF1 and Tfam (n = 6). (F) mRNA levels of Sirt1, Sirt3 and UCP1 (n = 6). Data are presented as the mean ± SD. For A-F, the statistical analysis was carried out by one-way ANOVA. *P < 0.05 vs the matched control.
Previous studies have demonstrated that bezafibrate could enhance the transcription of key fatty acid β-oxidation genes [51]. Therefore, the mRNA level of acyl-CoA oxidase 1 (ACOX1), carnitine palmitoyl-transferase 1A (CPT1A) and CD36 were detected. We found that mRNA levels of ACOX1, CPT1A and CD36 in the testis of DM mice were further increased by bezafibrate treatment (Fig. 5D). The mRNA levels of mitochondrial biogenesis indices including PPAR-γ co-activator-1 alpha (PGC1-α), Nfe2l1 (NRF1) and mitochondrial transcription factor A (Tfam) were also determined. The results indicated that the mRNA levels of PGC1-α, NRF1 and Tfam in the testis of DM mice were all increased by bezafibrate (Fig. 5E). Sirtuin 1 (Sirt1), Sirt3 and the uncoupling protein 1 (UCP1) are physiologically activated by fatty acids and mRNA levels of Sirt1, Sirt3 and UCP1 were determined. The results revealed the decreased mRNA levels of Sirt1 and Sirt3 in the testis of DM mice were increased by bezafibrate, however, the mRNA level of UCP1 was not influenced by bezafibrate (Fig. 5F).
Testicular ROS production, MDA content and carbonyl protein levels were increased in the testis of DM mice and decreased after bezafibrate treatment (Fig. 6A–C). The mRNA levels of CAT, SOD and GSH-Px were decreased in the DM group, and these pathological declines were prevented by the treatment of bezafibrate (Fig. 6D). CAT activity, the total SOD activity and GSH-Px activity were also decreased in DM group, and bezafibrate treatment could restore the activities of these antioxidant enzyme in the testis of DM mice (Fig. 6E–G).
Fig. 6.
Bezafibrate attenuated oxidative stress status in DM mice. (A) ROS production of the indicated groups (n = 6). (B) MDA content of the indicated groups (n = 6). (C) Carbonyl protein level of the indicated groups (n = 6). (D) mRNA levels of CAT, SOD and GSH-Px (n = 6). (E–G) CAT, SOD and GSH-Px activity (n = 6). Data are presented as the mean ± SD. For A-G, the statistical analysis was carried out by one-way ANOVA. *P < 0.05 vs the matched control.
4.6. The protective effects of bezafibrate on high glucose-induced sperm function impairment were ablated by PPARα deletion or inhibition
The results demonstrated that bezafibrate administration significantly improved sperm viability and sperm motility of DM mice (Fig. 7A and B). After GW6471 addition, the protective effects of bezafibrate on sperm viability and sperm motility were abrogated (Fig. 7A and B). Moreover, the cell viability of TM4 Sertoli cells and testosterone secretion of TM3 Leydig cells were both decreased by HG treatment. After bezafibrate addition, the testosterone concentration of TM3 Leydig cells was improved, while the cell viability of TM4 Sertoli cells was not influenced (Fig. 7C and D). After GW6471 addition, the protective effects of bezafibrate on testosterone secretion were abrogated (Fig. 7D). However, GSK0660 and GW9662 were shown to have no influence on sperm viability and sperm motility (Fig. 7E and F). We also used sperms separated from PPARα knockout mice to further confirm the role of PPARα on the protective effects of bezafibrate on sperm viability and sperm motility. The results demonstrated that the protection of bezafibrate on HG-induced reduction in sperm viability and sperm motility was abrogated after PPARα deletion (Fig. 7G and H).
Fig. 7.
The protective effects of bezafibrate on sperm viability and sperm motility were abrogated by PPARα deletion or inhibition. (A–B) Sperm viability and sperm motility of DM mice after PPARα inhibition (n = 6). (C) Sertoli cell viability of the indicated groups (n = 6). (D) T concentration of the indicated groups (n = 6). (E–F) Sperm viability and sperm motility of DM mice after PPARβ and PPARγ inhibition (n = 6). (G–H) Sperm viability and sperm motility of HG treated sperm after PPARα deletion (n = 6). The uncropped blots were listed in Supplementary Fig. 1. Data are presented as the mean ± SD. For E, the statistical analysis was carried out by Student's two-tailed t-test; for others, statistical analysis was carried out by one-way ANOVA. *P < 0.05 vs the matched control.
4.7. The protective effects of bezafibrate on spermatogenesis dysfunction induced by DM were abrogated by PPARα deletion
We also used PPARα knockout mice to further investigate the role of PPARα in the protective effects of bezafibrate on DM-induced spermatogenesis dysfunction. The results demonstrated that after PPARα deletion, the protective effects of bezafibrate on spermatogenesis function were ablated, as manifested by the alterations in testis weight, histological morphology, sperm viability and sperm motility as well as serum testosterone level were not changed by bezafibrate after PPARα deletion (Fig. 8A–E). TUNEL staining and caspase 3 activity detection also revealed that the anti-apoptotic effects of bezafibrate in testis were abolished by PPARα deletion (Fig. 8F and G). Testicular inflammation factors detection showed a similar result. There were no differences in testicular TNF-α and IL-6 between DM + KO and DM + bezafibrate + KO groups (Fig. 8H and I). Testicular ROS production, MDA content and SOD content were not further improved by bezafibrate after PPARα deletion (Fig. 8J-L).
Fig. 8.
The protective effects of bezafibrate on DM-induced spermatogenesis were abrogated by PPARα deletion. (A) Testis weight (n = 6). (B) The histological morphology of the indicated groups (n = 6). (C–D) Sperm viability and sperm motility (n = 6). (E) Serum testosterone concentration (n = 6). (F) TUNEL staining of the indicated groups (n = 6). (G) The testicular caspase 3 activity of the indicated groups (n = 6). (H–I) Testicular concentrations of TNF-α and IL-6 (n = 6). (J) Testicular ROS production (n = 6). (K–L) Testicular concentrations of MDA and SOD (n = 6). Data are presented as the mean ± SD. For A-L, the statistical analysis was carried out by one-way ANOVA. *P < 0.05 vs the matched control.
5. Discussion
DM is a metabolic disease that seriously endangers male fertility [9,52]. Thus, exploring new therapeutic approaches is of crucial importance for treating DM-induced male subfertility. Our present study indicated that bezafibrate administration could attenuate DM-induced male spermatogenesis deficiency (Fig. 2). Considering previous studies illustrated that adipokines participated in the process of male infertility [32,[53], [54], [55], [56]], we detected the adipokines level of DM mice after bezafibrate treatment and found that bezafibrate increased testicular adiponectin and irisin levels without affecting leptin and CTRP3 levels (Fig. 1G–J). We also demonstrated that bezafibrate could ameliorate cell apoptosis, decrease inflammatory factors accumulation and decrease testicular oxidative stress status (Fig. 3, Fig. 4, Fig. 5, Fig. 6). Using PPARα knockout mice or the specific inhibitors, we indicated that the protective effects of bezafibrate against DM-induced male subfertility were dependent on PPARα (Fig. 7, Fig. 8).
It has been reported that bezafibrate could reduce plasma TG and plasma NEFA effects [57,58]. The results of our study also demonstrated that the increased plasma TG and plasma NEFA in DM mice were decreased after bezafibrate treatment (Fig. 1B and C). Our results also indicated that bezafibrate decreased blood glucose in DM mice (Fig. 1 D), which was in accordance with a previous study [58]. Considering that adipokines also participate in metabolic related male infertility and the close relationship between bezafibrate and adipokines, the concentration of leptin, adiponectin, irisin, and CTRP3 were evaluated in this study. As an important adipokine, leptin has been reported to play an important role in male infertility [[59], [60], [61]]. Previous study has revealed that bezafibrate could decrease plasma leptin level significantly without affecting the level of leptin mRNA expression [62]. Inconsistent with these findings, our data suggested that bezafibrate had no impact on plasma and testis leptin levels in DM mice (Fig. 1 G). Adiponectin could improve testicular functions by increasing the expression of insulin receptors and decreasing oxidative stress [63]. It has been reported that bezafibrate could increase blood adiponectin levels in DM mice [64]. Consistent with these findings, we also found that the reduction of adiponectin in plasma and testis was prevented by bezafibrate treatment (Fig. 1H). Our previous study has demonstrated that irisin could ameliorate high-fat diet-induced spermatogenesis dysfunction via the activation of the AMPKα signaling pathway [56]. We also revealed that CTRP3 protected male mice against obesity-induced spermatogenesis deficiency through the Sirt1 pathway [32]. Our data in the present study suggested that bezafibrate had no impact on plasma and testis CTRP3 levels in DM mice but could increase both plasma and testicular irisin levels (Fig. 1I and J). The improvement of biochemical results and adipokines might partly explain the protection of bezafibrate against DM-induced male subfertility.
During the progression of diabetes mellitus, hyperglycemia promotes the generation of ROS that contributes to the development of diabetic complications [65,66]. Moreover, ROS-induced lipid peroxidation contributed to the development and progression of diabetes related complications [67]. Oxidative stress has been demonstrated to play an important role in DM-related spermatogenesis dysfunction [68]. It was also reported that cilostazol alleviated streptozotocin-induced testicular injury in rats by improving anti-oxidative capacity and attenuating lipid peroxidation in testicular tissue [69]. In line with this finding, we also found that bezafibrate could attenuate ROS production and oxidative stress in DM testis (Fig. 6). Hyperglycemia significantly reduced endogenous antioxidants, rendering the testis more vulnerable to DM-induced oxidative stimuli. In this aspect, the restoration of antioxidant systems by the treatment of bezafibrate might also contribute to the protection of bezafibrate against DM-induced male subfertility. In this study, we also found that bezafibrate increased the expression of energy metabolism genes and mitochondrial biogenesis in the testis of DM mice (Fig. 5). The attenuation of oxidative damage by bezafibrate may be secondarily mediated by increased mitochondrial biogenesis. And effects of PPARα activation on mitochondrial biogenesis have been observed in bezafibrate-treated developing rats with neural injury in the brain [70].
Inflammation played a critical role in DM-induced male subfertility [68,71]. Here, we also found that bezafibrate prevents DM-related phosphorylation of p65 and subsequent activation of inflammatory factors in the testis (Fig. 4 A-D), which was in agreement with other studies reported that bezafibrate inhibited NF-κB activity in neurometabolic disorder disease [72]. Activated NF-κB signaling pathway upregulated the transcription of NLRP3 inflammasome [73]. Previous studies reported that activated NLRP3 contributes to toxin-induced testicular hypoplasia, and ischaemia-induced testicular injury in mice [[74], [75], [76]]. Our recent study also demonstrated that NLRP3 deficiency could prevent obesity-related spermatogenesis impairment and preserve the function of the blood-testis barrier, demonstrating a maladaptive role for NLRP3 during the development of obesity-related spermatogenesis impairment [77]. Consistent with these findings, in this study, we also found that bezafibrate could inhibit NLRP3 activation and suppress the secretion of IL-1β and IL-18, thus improving the inflammatory microenvironment in the testis of diabetes mice (Fig. 4D–F). These data suggested that the attenuation of p65-NLRP3 activation and subsequent inflammation accumulation contributed to the protection of bezafibrate against DM-induced male subfertility.
To confirm that PPARs activation occurred in bezafibrate-treated testis, we assessed expression levels of the three PPAR isotypes, and found that there was an increase in PPARα after bezafibrate treatment (Fig. 5A and B). Consistent with PPARα activation being involved in fatty acid β-oxidation [78,79], we also found that bezafibrate treatment increased gene expression of ACOX1, CPT1A and CD36 in diabetic testis (Fig. 5 D). This finding was consistent with a previous report showing that bezafibrate administration also increased fatty acid oxidation and upregulated CPT1 expression in cytotoxic T lymphocytes [80]. To further confirm the role of PPARα in bezafibrate-provided protection, specific inhibitors as well as PPARα knockout mice were used. The protection of bezafibrate on spermatogenesis dysfunction was abrogated after PPARα deletion or inhibition (Fig. 7, Fig. 8), further confirming the critical role of PPARα in the protective effects provided by bezafibrate against diabetes-related spermatogenesis dysfunction.
Despite that this study illustrated that bezafibrate could ameliorate DM-induced spermatogenesis deficiency, the current study also has limitations. The mechanism underlying the improvement of vaginal plugs after bezafibrate treatment of DM mice was not documented in this study. Erectile dysfunction, which has been reported in diabetic mice, might participate in the reduction of sexuality of DM male mice [81,82]. And the reduction of plugs of DM mice was increased by bezafibrate might be due to the elevation of serum testosterone level and the subsequent improved sexuality. The specific target cell of bezafibrate was not fully clarified and the underlying mechanism which mediated the protective effects of bezafibrate still needs further investigation.
In conclusion, this study indicated a protective role of bezafibrate in diabetes-related spermatogenesis dysfunction by inhibiting inflammation and oxidative damage through PPARα activation. This study provided evidence that bezafibrate might be beneficial for diabetes-related male infertility.
Ethics statement
This work was approved by the Institutional Animal Care and Use Committee (IACUC) of Wuhan University Center for Animal (WP20210423).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Yang Mu: Project administration, Methodology, Investigation, Funding acquisition. Ling-Bo Luo: Project administration, Methodology, Investigation. Shu-juan Wu: Project administration, Methodology, Investigation. Yue Gao: Software, Formal analysis, Data curation. Xiao-lin Qin: Software, Formal analysis, Data curation. Jing Zhao: Writing – review & editing, Writing – original draft, Software, Formal analysis, Data curation. Qian Liu: Writing – review & editing, Writing – original draft, Project administration, Investigation. Jing Yang: Writing – review & editing, Visualization, Validation, Supervision, Investigation, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 82001609) and the Health Commission of Hubei Province scientific research project (WJ2021M146).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28284.
Contributor Information
Jing Zhao, Email: Agnes-zhaojing@whu.edu.cn.
Qian Liu, Email: drliuqian@whu.edu.cn.
Jing Yang, Email: dryangjing@whu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants [Journal Article; Meta-Analysis; Research Support, Non-U.S. Gov't] Lancet (N. Am. Ed.) 2016;387(10027) doi: 10.1016/S0140-6736(16)00618-8. 1513-30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang T.S., Wu T., Wu Y.D., Li X.H., Tan J., Shen C.H., et al. Long-term statins administration exacerbates diabetic nephropathy via ectopic fat deposition in diabetic mice [Journal Article; Research Support, Non-U.S. Gov't] Nat. Commun. 2023;14(1):390. doi: 10.1038/s41467-023-35944-z. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flyvbjerg A. The role of the complement system in diabetic nephropathy [Journal Article; Review] Nat. Rev. Nephrol. 2017;13(5):311–318. doi: 10.1038/nrneph.2017.31. eng. [DOI] [PubMed] [Google Scholar]
- 4.Qi C., Mao X., Zhang Z., Wu H. Classification and Differential diagnosis of diabetic nephropathy [journal article; review] J. Diabetes Res. 2017;2017 doi: 10.1155/2017/8637138. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechner J., O'Leary O.E., Stitt A.W. The pathology associated with diabetic retinopathy [Journal Article; Review] Vision Res. 2017;139:7–14. doi: 10.1016/j.visres.2017.04.003. eng. [DOI] [PubMed] [Google Scholar]
- 6.Huang T.S., Wu T., Wu Y.D., Li X.H., Tan J., Shen C.H., et al. Long-term statins administration exacerbates diabetic nephropathy via ectopic fat deposition in diabetic mice [Journal Article; Research Support, Non-U.S. Gov't] Nat. Commun. 2023;14(1):390. doi: 10.1038/s41467-023-35944-z. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thong E.P., Codner E., Laven J., Teede H. Diabetes: a metabolic and reproductive disorder in women [journal article; research support, non-U.S. Gov't; review] Lancet Diabetes Endocrinol. 2020;8(2):134–149. doi: 10.1016/S2213-8587(19)30345-6. eng. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi J., Dagur G., Warren K., Smith N.L., Sheynkin Y.R., Zumbo A., et al. The role of diabetes mellitus in sexual and reproductive health: an overview of pathogenesis, evaluation, and management [journal article; review] Curr. Diabetes Rev. 2017;13(6):573–581. doi: 10.2174/1573399813666161122124017. eng. [DOI] [PubMed] [Google Scholar]
- 9.Maresch C.C., Stute D.C., Alves M.G., Oliveira P.F., de Kretser D.M., Linn T. Diabetes-induced hyperglycemia impairs male reproductive function: a systematic review [Journal Article; Review; Systematic Review] Hum. Reprod. Update. 2018;24(1):86–105. doi: 10.1093/humupd/dmx033. eng. [DOI] [PubMed] [Google Scholar]
- 10.Yan X., Feng Y., Hao Y., Zhong R., Jiang Y., Tang X., et al. Gut-testis Axis: microbiota prime metabolome to increase sperm quality in young type 2 diabetes [journal article; research support, non-U.S. Gov't] Microbiol. Spectr. 2022;10(5) doi: 10.1128/spectrum.01423-22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parastesh M., Saremi A., Ahmadi A., Kaviani M. The effect of aerobic training on serum levels of adiponectin, hypothalamic-pituitary-gonadal Axis and sperm quality in diabetic rats. Urol. J. 2019;16(6):592–597. doi: 10.22037/uj.v0i0.4728. [Journal Article] eng. [DOI] [PubMed] [Google Scholar]
- 12.Oghbaei H., Fattahi A., Hamidian G., Sadigh-Eteghad S., Ziaee M., Mahmoudi J. A closer look at the role of insulin for the regulation of male reproductive function [Journal Article; Research Support, Non-U.S. Gov't; Review] Gen. Comp. Endocrinol. 2021;300 doi: 10.1016/j.ygcen.2020.113643. eng. [DOI] [PubMed] [Google Scholar]
- 13.Nna V.U., Bakar A., Ahmad A., Mohamed M. Diabetes-induced testicular oxidative stress, inflammation, and caspase-dependent apoptosis: the protective role of metformin [Journal Article] Arch. Physiol. Biochem. 2020;126(5):377–388. doi: 10.1080/13813455.2018.1543329. eng. [DOI] [PubMed] [Google Scholar]
- 14.Nna V.U., Abu B.A., Ahmad A., Eleazu C.O., Mohamed M. Oxidative stress, NF-kappaB-Mediated inflammation and apoptosis in the testes of streptozotocin-induced diabetic rats: combined protective effects of Malaysian propolis and metformin [journal article] Antioxidants. 2019;8(10) doi: 10.3390/antiox8100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ujah G., Emmanuel I.B., Ansa F., Ukoh A., Ani E.J., Osim E.E. Insulin and zinc Co-administration ameliorate diabetes mellitus-induced reproductive dysfunction in male rats [journal article] Niger. J. Physiol. Sci. 2022;37(1):49–58. doi: 10.54548/njps.v37i1.7. eng. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Guan T., Yang B., Chi Z., Wang Z.Y., Gu H.F. A novel role for zinc transporter 8 in the facilitation of zinc accumulation and regulation of testosterone synthesis in Leydig cells of human and mouse testicles [Journal Article; Research Support, Non-U.S. Gov't] Metabolism. 2018;88:40–50. doi: 10.1016/j.metabol.2018.09.002. eng. [DOI] [PubMed] [Google Scholar]
- 17.Sun N., Shen C., Zhang L., Wu X., Yu Y., Yang X., et al. Hepatic Kruppel-like factor 16 (KLF16) targets PPARalpha to improve steatohepatitis and insulin resistance [Journal Article; Research Support, Non-U.S. Gov't] Gut. 2021;70(11):2183–2195. doi: 10.1136/gutjnl-2020-321774. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawlak M., Lefebvre P., Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease [Journal Article; Review] J. Hepatol. 2015;62(3):720–733. doi: 10.1016/j.jhep.2014.10.039. eng. [DOI] [PubMed] [Google Scholar]
- 19.Yaribeygi H., Mohammadi M.T., Sahebkar A. PPAR-Alpha agonist improves hyperglycemia-induced oxidative stress in pancreatic cells by potentiating antioxidant defense system [journal article] Drug Res. 2018;68(6):355–360. doi: 10.1055/s-0043-121143. eng. [DOI] [PubMed] [Google Scholar]
- 20.Wang L., Cai Y., Jian L., Cheung C.W., Zhang L., Xia Z. Impact of peroxisome proliferator-activated receptor-alpha on diabetic cardiomyopathy [journal article; research support, non-U.S. Gov't; review] Cardiovasc. Diabetol. 2021;20(1):2. doi: 10.1186/s12933-020-01188-0. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu L., Liu C., Chang D.Y., Zhan R., Zhao M., Man L.S., et al. The attenuation of diabetic nephropathy by annexin A1 via regulation of lipid metabolism through the AMPK/PPARalpha/CPT1b pathway [journal article; research support, non-U.S. Gov't] Diabetes. 2021;70(10):2192–2203. doi: 10.2337/db21-0050. eng. [DOI] [PubMed] [Google Scholar]
- 22.Montaigne D., Butruille L., Staels B. PPAR control of metabolism and cardiovascular functions [journal article; research support, non-U.S. Gov't; review] Nat. Rev. Cardiol. 2021;18(12):809–823. doi: 10.1038/s41569-021-00569-6. eng. [DOI] [PubMed] [Google Scholar]
- 23.Franko A., Irmler M., Prehn C., Heinzmann S.S., Schmitt-Kopplin P., Adamski J., et al. Bezafibrate reduces elevated hepatic fumarate in insulin-deficient mice. Biomedicines. 2022;10(3) doi: 10.3390/biomedicines10030616. [Journal Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi T., Shirai K., Nagayama D., Nakamura S., Oka R., Tanaka S., et al. Bezafibrate ameliorates arterial stiffness assessed by cardio-ankle vascular index in hypertriglyceridemic patients with type 2 diabetes mellitus [journal article; randomized controlled trial] J. Atheroscler. Thromb. 2019;26(7):659–669. doi: 10.5551/jat.45799. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdullah F., Khan N.M., Agarwal R., Kamsani Y.S., Abd M.M., Bakar N.S., et al. Glutathione (GSH) improves sperm quality and testicular morphology in streptozotocin-induced diabetic mice [Journal Article; Research Support, Non-U.S. Gov't] Asian J. Androl. 2021;23(3):281–287. doi: 10.4103/aja.aja_81_20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei X., Huo P., Xie Y.J., Wang Y., Liu G., Tu H., et al. Dendrobium nobile Lindl polysaccharides improve testicular spermatogenic function in streptozotocin-induced diabetic rats [Journal Article; Research Support, Non-U.S. Gov't] Mol. Reprod. Dev. 2022;89(4):202–213. doi: 10.1002/mrd.23556. eng. [DOI] [PubMed] [Google Scholar]
- 27.Ma D., Hu L., Wang J., Luo M., Liang A., Lei X., et al. Nicotinamide mononucleotide improves spermatogenic function in streptozotocin-induced diabetic mice via modulating the glycolysis pathway [Journal Article] Acta Biochim. Biophys. Sin. 2022;54(9):1314–1324. doi: 10.3724/abbs.2022099. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franko A., Huypens P., Neschen S., Irmler M., Rozman J., Rathkolb B., et al. Bezafibrate improves insulin sensitivity and metabolic flexibility in STZ-induced diabetic mice [journal article; research support, non-U.S. Gov't] Diabetes. 2016;65(9):2540–2552. doi: 10.2337/db15-1670. eng. [DOI] [PubMed] [Google Scholar]
- 29.Ma Z.G., Yuan Y.P., Xu S.C., Wei W.Y., Xu C.R., Zhang X., et al. CTRP3 attenuates cardiac dysfunction, inflammation, oxidative stress and cell death in diabetic cardiomyopathy in rats [Journal Article; Research Support, Non-U.S. Gov't] Diabetologia. 2017;60(6):1126–1137. doi: 10.1007/s00125-017-4232-4. eng. [DOI] [PubMed] [Google Scholar]
- 30.Nakano S., Inada Y., Masuzaki H., Tanaka T., Yasue S., Ishii T., et al. Bezafibrate regulates the expression and enzyme activity of 11beta-hydroxysteroid dehydrogenase type 1 in murine adipose tissue and 3T3-L1 adipocytes [Journal Article; Research Support, Non-U.S. Gov't] Am. J. Physiol. Endocrinol. Metab. 2007;292(4) doi: 10.1152/ajpendo.00340.2006. E1213-22. eng. [DOI] [PubMed] [Google Scholar]
- 31.Mu Y., Yin T.L., Huang X.X., Hu X., Yin L., Yang J. Sulforaphane ameliorates high-fat diet-induced spermatogenic deficiency in micedagger [Journal Article; Research Support, Non-U.S. Gov't] Biol. Reprod. 2019;101(1):223–234. doi: 10.1093/biolre/ioz067. eng. [DOI] [PubMed] [Google Scholar]
- 32.Mu Y., Yin T.L., Yin L., Hu X., Yang J. CTRP3 attenuates high-fat diet-induced male reproductive dysfunction in mice [Journal Article; Research Support, Non-U.S. Gov't] Clin. Sci. (Lond.) 2018;132(8):883–899. doi: 10.1042/CS20180179. eng. [DOI] [PubMed] [Google Scholar]
- 33.Mu Y., Yan W.J., Yin T.L., Zhang Y., Li J., Yang J. Diet-induced obesity impairs spermatogenesis: a potential role for autophagy [Journal Article; Research Support, Non-U.S. Gov't] Sci. Rep. 2017;7 doi: 10.1038/srep43475. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Memudu A.E., Duru F.I. A Comparative study on the effects of Yaji (Suya Meat sauce) and its spice constituents on the male reproductive profile of adult male Sprague Dawley rats [Journal Article] JBRA. Assist. Reprod. 2021;25(4):509–523. doi: 10.5935/1518-0557.20210020. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portela J.M., Tavares R.S., Mota P.C., Ramalho-Santos J., Amaral S. High glucose concentrations per se do not adversely affect human sperm function in vitro [Journal Article; Research Support, Non-U.S. Gov't] Reproduction. 2015;150(1):77–84. doi: 10.1530/REP-15-0100. eng. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S., Liu Q., Wright M., Garvin J., Rarick K., Harder D. High glucose conditioned neonatal astrocytes results in impaired mitogenic activity in cerebral microvessel endothelial cells in co-culture [Journal Article] Heliyon. 2019;5(5) doi: 10.1016/j.heliyon.2019.e01795. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu S.C., Ma Z.G., Wei W.Y., Yuan Y.P., Tang Q.Z. Bezafibrate attenuates pressure overload-induced cardiac hypertrophy and fibrosis [journal article] PPAR Res. 2017;2017 doi: 10.1155/2017/5789714. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H., Luo Q., Lu X., Yin N., Zhou D., Zhang L., et al. Retraction Note: effects of hPMSCs on granulosa cell apoptosis and AMH expression and their role in the restoration of ovary function in premature ovarian failure mice [Retraction of Publication] Stem Cell Res. Ther. 2022;13(1):504. doi: 10.1186/s13287-022-03183-6. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M., Wang Y., Huan Z., Liu Y., Zhang W., Kong D., et al. FSH modulated cartilage ECM metabolism by targeting the PKA/CREB/SOX9 pathway [Journal Article] J. Bone Miner. Metabol. 2021;39(5):769–779. doi: 10.1007/s00774-021-01232-3. eng. [DOI] [PubMed] [Google Scholar]
- 40.Ma Z.G., Yuan Y.P., Zhang X., Xu S.C., Kong C.Y., Song P., et al. C1q-tumour necrosis factor-related protein-3 exacerbates cardiac hypertrophy in mice [Journal Article; Research Support, Non-U.S. Gov't] Cardiovasc. Res. 2019;115(6):1067–1077. doi: 10.1093/cvr/cvy279. eng. [DOI] [PubMed] [Google Scholar]
- 41.Hallmann A., Michnowska A., Chomiczewska A., Lipinski M., Smolarz K. Bivalves transmissible neoplasia: biochemical aspects of contagious cancer in a clam Macoma balthica [journal article] Cell. Physiol. Biochem. 2022;56(6):629–643. doi: 10.33594/000000587. eng. [DOI] [PubMed] [Google Scholar]
- 42.Elfassy Y., Bastard J.P., McAvoy C., Fellahi S., Dupont J., Levy R. Adipokines in semen: physiopathology and effects on spermatozoas. INT J ENDOCRINOL. 2018;2018 doi: 10.1155/2018/3906490. [Journal Article; Review] eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elfassy Y., McAvoy C., Fellahi S., Dupont J., Feve B., Levy R., et al. Seminal plasma adipokines: involvement in human reproductive functions [Journal Article; Review] Eur. Cytokine Netw. 2017;28(4) doi: 10.1684/ecn.2018.0403. 141-50. eng. [DOI] [PubMed] [Google Scholar]
- 44.Syriou V., Papanikolaou D., Kozyraki A., Goulis D.G. Cytokines and male infertility [journal article; review] Eur. Cytokine Netw. 2018;29(3):73–82. doi: 10.1684/ecn.2018.0412. eng. [DOI] [PubMed] [Google Scholar]
- 45.Condorelli R.A., La Vignera S., Mongioi L.M., Alamo A., Calogero A.E. Diabetes mellitus and infertility: different pathophysiological effects in type 1 and type 2 on sperm function [journal article] Front. Endocrinol. 2018;9:268. doi: 10.3389/fendo.2018.00268. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yelumalai S., Giribabu N., Karim K., Omar S.Z., Salleh N.B. In vivo administration of quercetin ameliorates sperm oxidative stress, inflammation, preserves sperm morphology and functions in streptozotocin-nicotinamide induced adult male diabetic rats [Journal Article] Arch. Med. Sci. 2019;15(1):240–249. doi: 10.5114/aoms.2018.81038. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnabei L., Laplantine E., Mbongo W., Rieux-Laucat F., Weil R. NF-kappaB: at the borders of autoimmunity and inflammation [journal article; research support, non-U.S. Gov't; review] Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.716469. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chawla M., Roy P., Basak S. Role of the NF-kappaB system in context-specific tuning of the inflammatory gene response [Journal Article; Research Support, Non-U.S. Gov't; Review] Curr. Opin. Immunol. 2021;68:21–27. doi: 10.1016/j.coi.2020.08.005. eng. [DOI] [PubMed] [Google Scholar]
- 49.Hong Y., Zhou Y., Shen L., Wei Y., Long C., Fu Y., et al. Exposure to DEHP induces testis toxicity and injury through the ROS/mTOR/NLRP3 signaling pathway in immature rats [Journal Article] Ecotoxicol. Environ. Saf. 2021;227 doi: 10.1016/j.ecoenv.2021.112889. eng. [DOI] [PubMed] [Google Scholar]
- 50.Fouad A.A., Abdel-Aziz A.M., Hamouda A. Diacerein downregulates NLRP3/caspase-1/IL-1beta and IL-6/STAT3 pathways of inflammation and apoptosis in a rat model of cadmium testicular toxicity [journal article] Biol. Trace Elem. Res. 2020;195(2):499–505. doi: 10.1007/s12011-019-01865-6. eng. [DOI] [PubMed] [Google Scholar]
- 51.Frambach S., van de Wal M., van den Broek P., Smeitink J., Russel F., de Haas R., et al. Effects of clofibrate and KH176 on life span and motor function in mitochondrial complex I-deficient mice [Journal Article; Research Support, Non-U.S. Gov't] Biochim. Biophys. Acta, Mol. Basis Dis. 2020;1866(6) doi: 10.1016/j.bbadis.2020.165727. eng. [DOI] [PubMed] [Google Scholar]
- 52.Glazer C.H., Bonde J.P., Giwercman A., Vassard D., Pinborg A., Schmidt L., et al. Risk of diabetes according to male factor infertility: a register-based cohort study [Comparative Study; Journal Article; Research Support, Non-U.S. Gov't] Hum. Reprod. 2017;32(7):1474–1481. doi: 10.1093/humrep/dex097. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malik I.A., Durairajanayagam D., Singh H.J. Leptin and its actions on reproduction in males [journal article; research support, non-U.S. Gov't; review] Asian J. Androl. 2019;21(3):296–299. doi: 10.4103/aja.aja_98_18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choubey M., Ranjan A., Bora P.S., Baltazar F., Martin L.J., Krishna A. Role of adiponectin as a modulator of testicular function during aging in mice [Journal Article; Research Support, Non-U.S. Gov't] Biochim. Biophys. Acta, Mol. Basis Dis. 2019;1865(2):413–427. doi: 10.1016/j.bbadis.2018.11.019. eng. [DOI] [PubMed] [Google Scholar]
- 55.Yardimci A., Ulker N., Bulmus O., Sahin E., Alver A., Gungor I.H., et al. Irisin improves high-fat diet-induced sexual dysfunction in obese male rats [journal article; research support, non-U.S. Gov't] Neuroendocrinology. 2022;112(11):1087–1103. doi: 10.1159/000523689. eng. [DOI] [PubMed] [Google Scholar]
- 56.Mu Y., Dai H.G., Luo L.B., Yang J. Irisin alleviates obesity-related spermatogenesis dysfunction via the regulation of the AMPKalpha signalling pathway [Journal Article] Reprod. Biol. Endocrinol. 2021;19(1):135. doi: 10.1186/s12958-021-00821-1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi T., Shirai K., Nagayama D., Nakamura S., Oka R., Tanaka S., et al. Bezafibrate ameliorates arterial stiffness assessed by cardio-ankle vascular index in hypertriglyceridemic patients with type 2 diabetes mellitus [journal article; randomized controlled trial] J. Atheroscler. Thromb. 2019;26(7):659–669. doi: 10.5551/jat.45799. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franko A., Neschen S., Rozman J., Rathkolb B., Aichler M., Feuchtinger A., et al. Bezafibrate ameliorates diabetes via reduced steatosis and improved hepatic insulin sensitivity in diabetic TallyHo mice [Journal Article; Research Support, Non-U.S. Gov't] Mol. Metab. 2017;6(3):256–266. doi: 10.1016/j.molmet.2016.12.007. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khodamoradi K., Parmar M., Khosravizadeh Z., Kuchakulla M., Manoharan M., Arora H. The role of leptin and obesity on male infertility [Journal Article; Research Support, Non-U.S. Gov't; Review] Curr. Opin. Urol. 2020;30(3):334–339. doi: 10.1097/MOU.0000000000000762. eng. [DOI] [PubMed] [Google Scholar]
- 60.Amjad S., Baig M., Zahid N., Tariq S., Rehman R. Association between leptin, obesity, hormonal interplay and male infertility. Andrologia. 2019;51(1) doi: 10.1111/and.13147. [Journal Article] eng. [DOI] [PubMed] [Google Scholar]
- 61.Childs G.V., Odle A.K., MacNicol M.C., MacNicol A.M. The importance of leptin to reproduction [journal article; research support, N.I.H., extramural; review] Endocrinology. 2021;162(2) doi: 10.1210/endocr/bqaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harada N., Kusuyama A., Morishima M., Okada K., Takahashi A., Nakaya Y. Bezafibrate improves bacterial lipopolysaccharide-induced dyslipidemia and anorexia in rats [Journal Article] Metabolism. 2007;56(4):517–522. doi: 10.1016/j.metabol.2006.11.011. eng. [DOI] [PubMed] [Google Scholar]
- 63.Choubey M., Ranjan A., Bora P.S., Krishna A. Protective role of adiponectin against testicular impairment in high-fat diet/streptozotocin-induced type 2 diabetic mice. Biochimie. 2020;168:41–52. doi: 10.1016/j.biochi.2019.10.014. [Journal Article] eng. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki Y., Shimada T., Iizuka S., Suzuki W., Makihara H., Teraoka R., et al. Effects of bezafibrate in nonalcoholic steatohepatitis model mice with monosodium glutamate-induced metabolic syndrome [Journal Article; Research Support, Non-U.S. Gov't] Eur. J. Pharmacol. 2011;662(1–3):1–8. doi: 10.1016/j.ejphar.2011.04.051. eng. [DOI] [PubMed] [Google Scholar]
- 65.An Y., Zhang H., Wang C., Jiao F., Xu H., Wang X., et al. Activation of ROS/MAPKs/NF-kappaB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis [Journal Article; Research Support, Non-U.S. Gov't] Faseb. J. 2019;33(11):12515–12527. doi: 10.1096/fj.201802805RR. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hegyi B., Borst J.M., Bailey L., Shen E.Y., Lucena A.J., Navedo M.F., et al. Hyperglycemia regulates cardiac K(+) channels via O-GlcNAc-CaMKII and NOX2-ROS-PKC pathways [journal article; research support, N.I.H., extramural] Basic Res. Cardiol. 2020;115(6):71. doi: 10.1007/s00395-020-00834-8. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang Q., Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications [Journal Article; Research Support, Non-U.S. Gov't; Review] Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101799. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nasiri K., Akbari A., Nimrouzi M., Ruyvaran M., Mohamadian A. Safflower seed oil improves steroidogenesis and spermatogenesis in rats with type II diabetes mellitus by modulating the genes expression involved in steroidogenesis, inflammation and oxidative stress [Journal Article] J. Ethnopharmacol. 2021;275 doi: 10.1016/j.jep.2021.114139. eng. [DOI] [PubMed] [Google Scholar]
- 69.Mohamed M.Z., Hafez H.M., Zenhom N.M., Mohammed H.H. Cilostazol alleviates streptozotocin-induced testicular injury in rats via PI3K/Akt pathway. Life Sci. 2018;198:136–142. doi: 10.1016/j.lfs.2018.02.038. [Journal Article] eng. [DOI] [PubMed] [Google Scholar]
- 70.Da R.N., Parmeggiani B., Da R.M., Glanzel N.M., de Moura A.L., Wajner M., et al. Bezafibrate in vivo administration prevents 3-methylglutaric acid-induced impairment of redox status, mitochondrial biogenesis, and neural injury in brain of developing rats [journal article] Neurotox. Res. 2019;35(4):809–822. doi: 10.1007/s12640-019-00019-9. eng. [DOI] [PubMed] [Google Scholar]
- 71.Khalil A., Giribabu N., Yelumalai S., Shahzad H., Kilari E.K., Salleh N. Myristic acid defends against testicular oxidative stress, inflammation, apoptosis: restoration of spermatogenesis, steroidogenesis in diabetic rats [Journal Article] Life Sci. 2021;278 doi: 10.1016/j.lfs.2021.119605. eng. [DOI] [PubMed] [Google Scholar]
- 72.Seminotti B., Brondani M., Ribeiro R.T., Leipnitz G., Wajner M. Disturbance of mitochondrial dynamics, endoplasmic reticulum-mitochondria crosstalk, redox homeostasis, and inflammatory response in the brain of glutaryl-CoA dehydrogenase-deficient mice: neuroprotective effects of bezafibrate [journal article] Mol. Neurobiol. 2022;59(8):4839–4853. doi: 10.1007/s12035-022-02887-3. eng. [DOI] [PubMed] [Google Scholar]
- 73.Peng L., Wen L., Shi Q.F., Gao F., Huang B., Meng J., et al. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-kappaB/NLRP3-mediated epithelial-mesenchymal transition and inflammation [Journal Article] Cell Death Dis. 2020;11(11):978. doi: 10.1038/s41419-020-03178-2. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y., Su Y., Zhou T., Hu Z., Wei J., Wang W., et al. Activation of the NLRP3 inflammasome pathway by prokineticin 2 in testicular macrophages of uropathogenic Escherichia coli- induced orchitis [journal article; research support, non-U.S. Gov't] Front. Immunol. 2019;10:1872. doi: 10.3389/fimmu.2019.01872. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Minutoli L., Antonuccio P., Irrera N., Rinaldi M., Bitto A., Marini H., et al. NLRP3 inflammasome involvement in the organ damage and impaired spermatogenesis induced by testicular ischemia and reperfusion in mice [journal article; research support, non-U.S. Gov't] J. Pharmacol. Exp. Ther. 2015;355(3):370–380. doi: 10.1124/jpet.115.226936. eng. [DOI] [PubMed] [Google Scholar]
- 76.Zhou Y., Ma T., Yan M., Meng X., Wu J., Ding J., et al. Exposure of DBP in gestation induces inflammation of testicular Sertoli cells in progeny by activating NLRP3 inflammasomes [Journal Article] Sci. Total Environ. 2020;707 doi: 10.1016/j.scitotenv.2019.136139. eng. [DOI] [PubMed] [Google Scholar]
- 77.Mu Y., Yin T.L., Zhang Y., Yang J., Wu Y.T. Diet-induced obesity impairs spermatogenesis: the critical role of NLRP3 in Sertoli cells. Inflamm. Regen. 2022;42(1):24. doi: 10.1186/s41232-022-00203-z. [Journal Article] eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naiman S., Huynh F.K., Gil R., Glick Y., Shahar Y., Touitou N., et al. SIRT6 promotes hepatic beta-oxidation via activation of PPARalpha [journal article; research support, N.I.H., extramural; research support, non-U.S. Gov't] Cell Rep. 2019;29(12):4127–4143. doi: 10.1016/j.celrep.2019.11.067. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Q., Kong X., Yuan H., Guan H., Li Y., Niu Y. Mangiferin improved palmitate-induced-insulin resistance by promoting free fatty acid metabolism in HepG2 and C2C12 cells via PPARalpha: mangiferin improved insulin resistance. J. Diabetes Res. 2019;2019 doi: 10.1155/2019/2052675. [Journal Article] eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chowdhury P.S., Chamoto K., Kumar A., Honjo T. PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8(+) T cells and facilitates anti-PD-1 therapy [journal article; research support, non-U.S. Gov't] Cancer Immunol. Res. 2018;6(11):1375–1387. doi: 10.1158/2326-6066.CIR-18-0095. eng. [DOI] [PubMed] [Google Scholar]
- 81.Anita L., Yin G.N., Hong S.S., Kang J.H., Gho Y.S., Suh J.K., et al. Pericyte-derived extracellular vesicle-mimetic nanovesicles ameliorate erectile dysfunction via lipocalin 2 in diabetic mice [Journal Article; Research Support, Non-U.S. Gov't] Int. J. Biol. Sci. 2022;18(9):3653–3667. doi: 10.7150/ijbs.72243. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yin G.N., Kim D.K., Kang J.I., Im Y., Lee D.S., Han A.R., et al. Latrophilin-2 is a novel receptor of LRG1 that rescues vascular and neurological abnormalities and restores diabetic erectile function [Journal Article; Research Support, Non-U.S. Gov't] Exp. Mol. Med. 2022;54(5):626–638. doi: 10.1038/s12276-022-00773-5. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.