Fig 5.
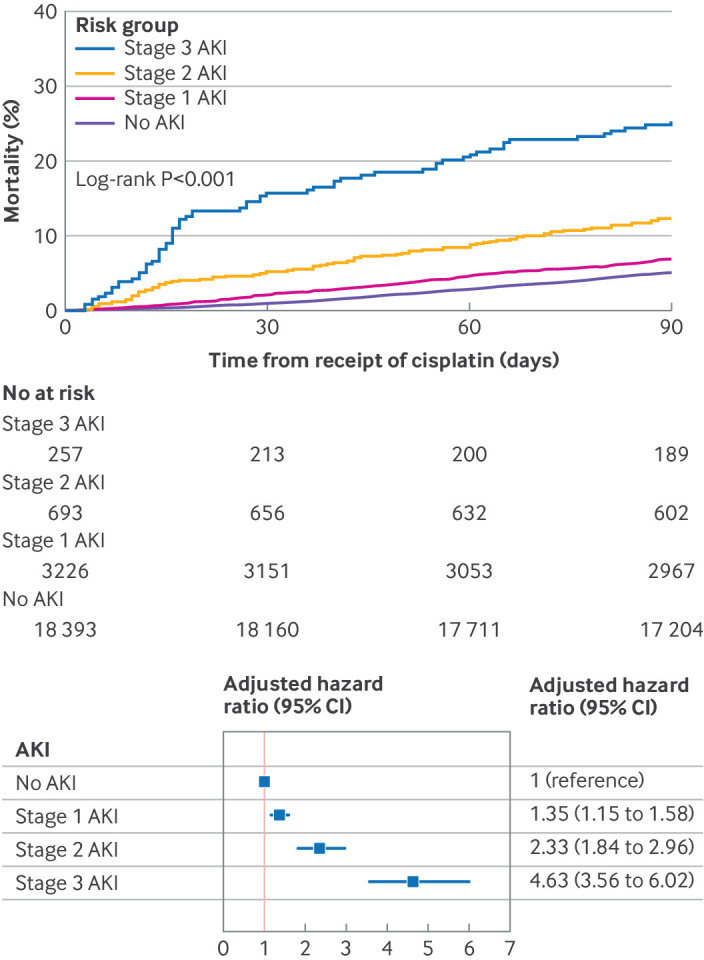
CP-AKI severity and mortality. Top panel shows cumulative incidence of death in the first 90 days after cisplatin was administered according to CP-AKI stage. CP-AKI severity was categorized into four groups according to stage of AKI: no AKI, stage 1 AKI (an increase in serum creatinine ≥26.5 µmol/L or a 1.5-1.9-fold increase in serum creatinine level), stage 2 AKI (2-2.9-fold increase in serum creatinine), or stage 3 AKI (≥3-fold increase in serum creatinine, or kidney replacement therapy), each assessed within 14 days after cisplatin was administered. Bottom panel shows a multivariable Cox model for 90 day survival according to CP-AKI stage. The model was adjusted for age, male sex, body mass index, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, current or former smoker status, serum creatinine level, hemoglobin level, white blood cell count, platelet count, serum albumin level, serum magnesium level, cisplatin dose, and concomitant nephrotoxic chemotherapy (binary variable that included pemetrexed, cetuximab, ifosfamide, or immune checkpoint inhibitors within 30 days before a first dose of intravenous cisplatin). AKI=acute kidney injury; CI=confidence interval; CP-AKI=cisplatin associated acute kidney injury
