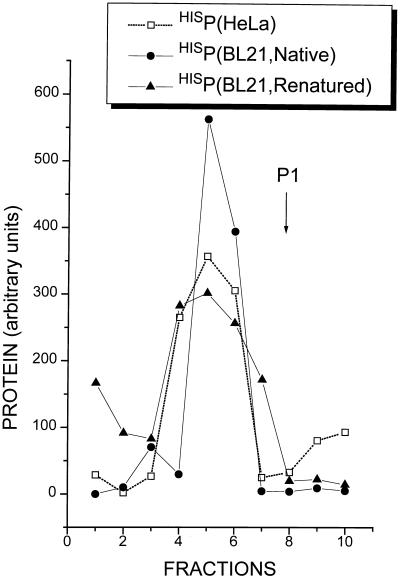
FIG. 2.
Sedimentation analysis of bacterially expressed HisP protein. E. coli BL21 transformed with pT7-7 HISP was grown in L broth supplemented with 0.3% glucose at 37°C to an optical density at 600 nm of 0.7. Gene expression was then induced by the addition of 1 mM IPTG, followed 90 min later by the addition of rifampin (200 μg/ml). Incubation was then continued for a further 4 h. Bacteria were pelleted and resuspended in lysis buffer (20 mM Tris-HCl [pH 8.0], 100 mM NaCl) containing either 1% NP-40 (native conditions) or 8 M urea (denaturing conditions). HisP was purified and renatured as described in Materials and Methods. These proteins were centrifuged on linear 5 to 20% glycerol gradients (40,000 rpm, 22 h, 4°C in a SW60 rotor; see reference 9) along with a cytoplasmic extract prepared from vTF7-3-infected HeLa cells transfected with the same plasmid clone. Gradients were fractionated, and aliquots from each fraction were analyzed by immunoblotting with the anti-P1.180 monoclonal antibody with an enhanced chemiluminescence light detection system. The amount of protein in each fraction was determined by densitometry and plotted (sedimentation was from right to left). P1 refers to the position of HAPΔ344-411 (a monomeric P protein [9]) sedimented on a parallel gradient.