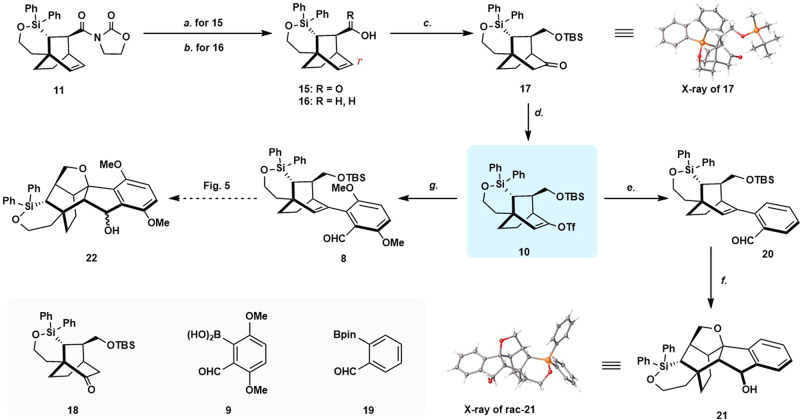
Fig. 4. Synthesis of hexacyclic intermediate 22.
Reagents and conditions: a LiOH (3.0 equiv), H2O2 (8.8 equiv), THF-H2O (v:v = 2:1), 0 °C to RT, 94%; b LiBH4 (4.0 equiv), THF, 0 °C, 85%; c i. BH3·THF (5.0 equiv), THF, ‒30 °C to RT, NaBO3 ·4H2O (10.0 equiv). ii. TBSCl (3.0 equiv), imid (5.0 equiv), DCM, RT. iii. DMP (2.0 equiv), NaHCO3 (10 equiv), RT, 69% for 17, 20% for 18; d KHMDS (1.4 equiv), PhNTf2 (1.4 equiv), THF, −78 oC, 94%; e 19 (2.0 equiv), Pd(dppf)Cl2 (0.1 equiv), K3CO3 (3.0 equiv), DMSO, 80 °C, 96%; f HCl (2 M in ethyl acetate) (10.0 equiv), DCM, ‒10 °C, 89%; g 9 (2.0 equiv), Pd(dppf)Cl2 (0.1 equiv), S-Phos (0.2 equiv), K3PO4 (3.0 equiv), DMF, 80 °C, 69%. THF tetrahydrofuran, TBSCl tert-Butyldimethylsilyl chloride, imid Imidazole, DMP Dess–Martin periodinane, KHMDS potassium bis(trimethylsilyl)amide, PhNTf2 N-phenyl-bis(trifluoromethanesulfonimide), S-Phos 2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl, DMF N, N-dimethylformamide.