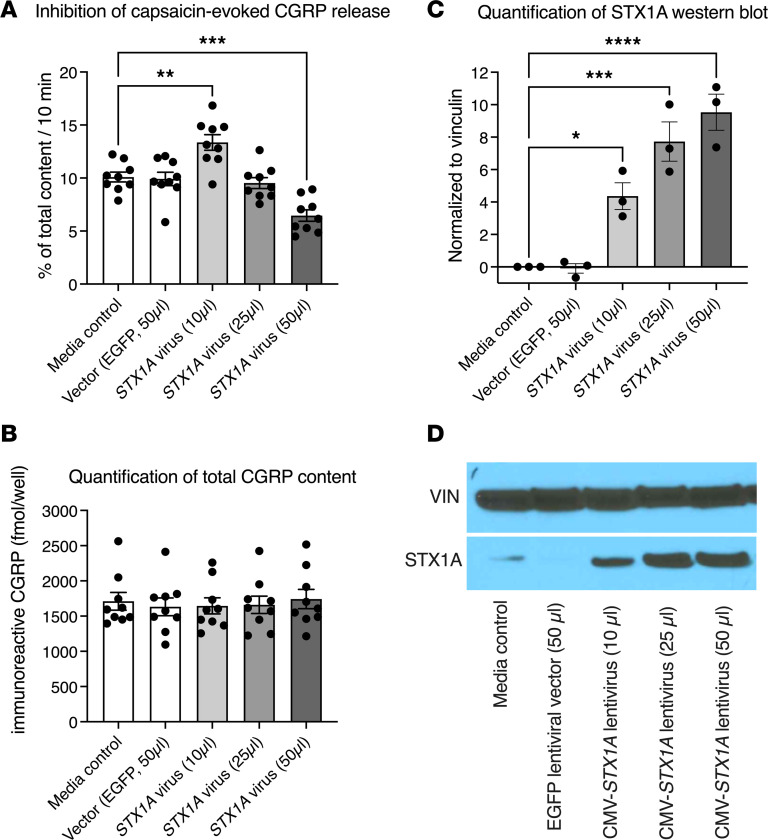
Figure 5. Overexpression of STX1A inhibits capsaicin-induced CGRP release from rat primary DRG neuronal culture.
Capsaicin (30 nM) activates the TRPV1 ion channel, causing depolarization and calcium influx, which triggers neuropeptide release (CGRP) from the TRPV1-expressing neurons on the culture plate. The dose of capsaicin was chosen from a capsaicin dose and vector concentration pilot study (Supplemental Figure 1); 1 μL of virus preparation equals 1 × 105 transducing units. Bars represent mean ± SEM and individual points are shown. (A) No difference is seen between control (culture medium, no vector) and vector only (expressing EGFP from the cytomegalovirus promoter). Ascending amounts of vector first produce an increase, and then a decrease, in capsaicin-evoked CGRP release (see also Supplemental Figure 1). (B) Addition of vector at any of the amounts used did not impair integrity of the primary cultured neurons. Loss of neurons from the plate would yield a decrease in total CGRP content. (C) Western blot analysis of STX1A content. Ascending amounts of vector produce significant, progressive increases in STX1A immunoreactive protein. (D) Photograph of the Western blot. Vinculin (VIN) was used to assess protein loading. Note that the basal amount of STX1A in the cultures is low. The increase from vector-generated STX1A may explain the initial increase in CGRP release with 10 μL vector. Release is then inhibited at 25 and 50 μL of vector. Statistics were performed by 1-way ANOVA followed by Dunnett’s post hoc test (GraphPad Prism 10) to test for differences in each group relative to the media control; *, **, ***, **** indicate significant differences from medium alone; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n = 9 primary cultures/condition.