Table 3.
Clinical application of combination therapy: BLIs and antibiotics.
| β-Lactamase Inhibitor | Chemical Structure |
Company | Clinical Trial Phase | Representative Combination | Indication |
|---|---|---|---|---|---|
| Clavulanic acid |
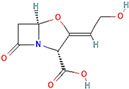
|
GlaxoSmithKline (London, the UK) | Phase 4 | Amoxicillin–Clavulanic Acid | Bacteremia (NCT02783404) Chronic Bronchitis (NCT00656747) Effects on Gut Microbiota (NCT04084106) Acute Otitis Media (NCT00644943) |
| Sulbactam |
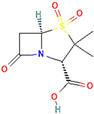
|
Pfizer Inc. (New York, NY, USA) | Phase 4 | Ampicillin–Sulbactam | Cesarean Section (NCT01138852) Intra-Abdominal Infection (NCT00952796) Acinetobacter Pneumonia (NCT05922124) Skin Infections (NCT00368537) |
| Tazobactam |
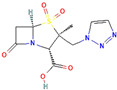
|
Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan) | Phase 4 | Piperacillin–Tazobactam Ceftolozane–Tazobactam |
Bloodstream Infections (NCT05355350) Febrile Neutropenia (NCT04233996) Diabetic Foot Infections (NCT00044746) Early Phase of Severe Sepsis and Septic Shock (NCT02730624) Cystic Fibrosis and Bronchiectasis (NCT06035055) |
| Enmetazobactam |
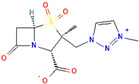
|
Allecra Therapeutics (Saint Louis, MO, USA) | Phase 3 Phase 2 |
Cefepime–Enmetazobactam | Urinary Tract Infection Complicated (NCT03687255, NCT05826990, NCT03680612) |
| Avibactam |
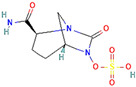
|
Pfizer Inc. (New York, NY, USA) | Phase 4 | Ceftazidime–Avibactam | Cystic Fibrosis (NCT02504827) Hospital-Acquired Pneumonia (NCT04774094) Urinary Tract Infection and Acute Pyelonephritis (NCT04882085) |
| Relebactam |
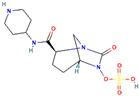
|
Merck Sharp & Dohme LLC (Rahway, NJ, USA) | Phase 4 | Imipenem–Cilastatin–Relebactam | Cystic Fibrosis and Bacterial Pneumonia (NCT05561764) Obesity and Critical Illness (NCT05146154) |
| Nacubactam |
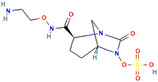
|
F. Hoffmann-La Roche, Ltd. (Basel, Switzerland) | Phase 1 | Meropenem–Nacubactam | Gram-Negative Bacterial Infections (NCT03182504) |
| Zidebactam |
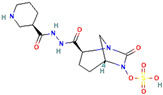
|
Medpace, Inc. (Cincinnati, OH, USA) | Phase 3 | Cefepime–Zidebactam | Complicated Urinary Tract Infections and Acute Pyelonephritis (NCT04979806) |
| Durlobactam |
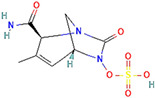
|
Entasis Therapeutics Holdings Inc. (Waltham, MA, USA) | Phase 3 Phase 2 |
Sulbactam–Durlobactam | Hospital-Acquired Bacterial Pneumonia (NCT03894046) Complicated Urinary Tract Infections and Acute Pyelonephritis (NCT03445195) |
| Funobactam |
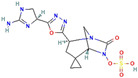
|
Evopoint Biosciences Inc. (Suzhou, China) | Phase 3 | Imipenem–Funobactam | Complicated Urinary Tract Infection Including Acute Pyelonephritis (NCT05204368) Hospital-Acquired Bacterial Pneumonia or Ventilator-Associated Bacterial Pneumonia (NCT05204563) |
| Vaborbactam |
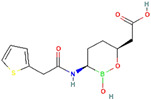
|
Melinta Therapeutics, Inc. (Parsippany, NJ, USA) | Phase 3 | Meropenem–Vaborbactam | Complicated Urinary Tract Infection and Acute Pyelonephritis (NCT02166476) Hospital-Acquired Bacterial Pneumonia (NCT02168946) |
| Taniborbactam |
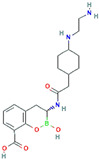
|
Venatorx Pharmaceuticals, Inc. (Malvern, PA, USA) | Phase 3 Phase 1 |
Cefepime–Taniborbactam | Complicated Urinary Tract Infection and Acute Pyelonephritis (NCT03840148) Pharmacokinetics (NCT04951505) |
| Xeruborbactam |
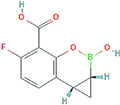
|
Qpex Biopharma, Inc. (San Diego, CA, USA) | Phase 1 | QPX2014–Xeruborbactam Ceftibuten–Xeruborbactam |
Pharmacokinetics and Side Effects (NCT05072444, NCT04380207) Bacterial Infections (NCT06079775) |
