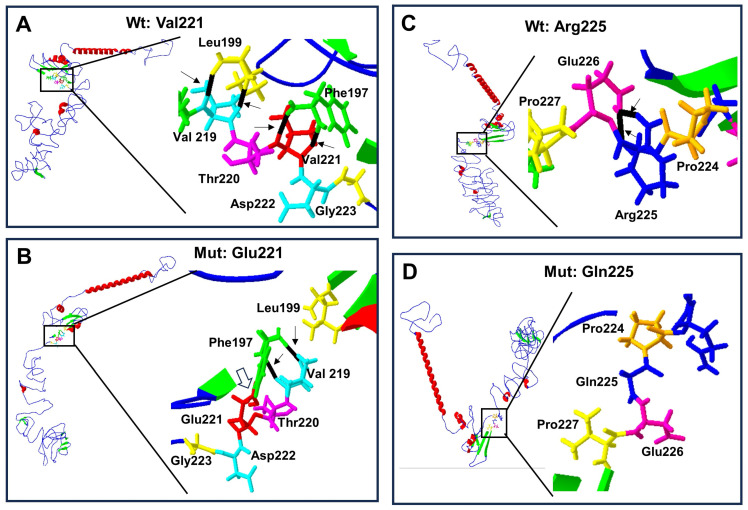
Figure 5.
Protein structure prediction of p.Val221Glu and p.Arg225Gln-mutated human RUNX2. Wild-type protein ((A) and (C) top left) acquires a folded conformation in the mutated proteins (B) and (D) down left), respectively. The α-helix (red), β-leaf (green), and coils/loops (blue) are shown. Enlarged image shows that (A) the p.Val221 (red) has two bridges of hydrogen linked with p.Phe197 (green), while (B) p.Glu221 (red) loses the two hydrogen bonds with p.Phe197 but acquires a covalent bond between them (open arrow); additionally, p.Phe197 links two hydrogens bonds with p.Val219 (light blue). Enlarged image: (C) the p.Arg225 (blue) has two hydrogen bonds linked with p.Gln226 (pink), while (D) p.Gln225 (blue) loses its two hydrogen bonds.