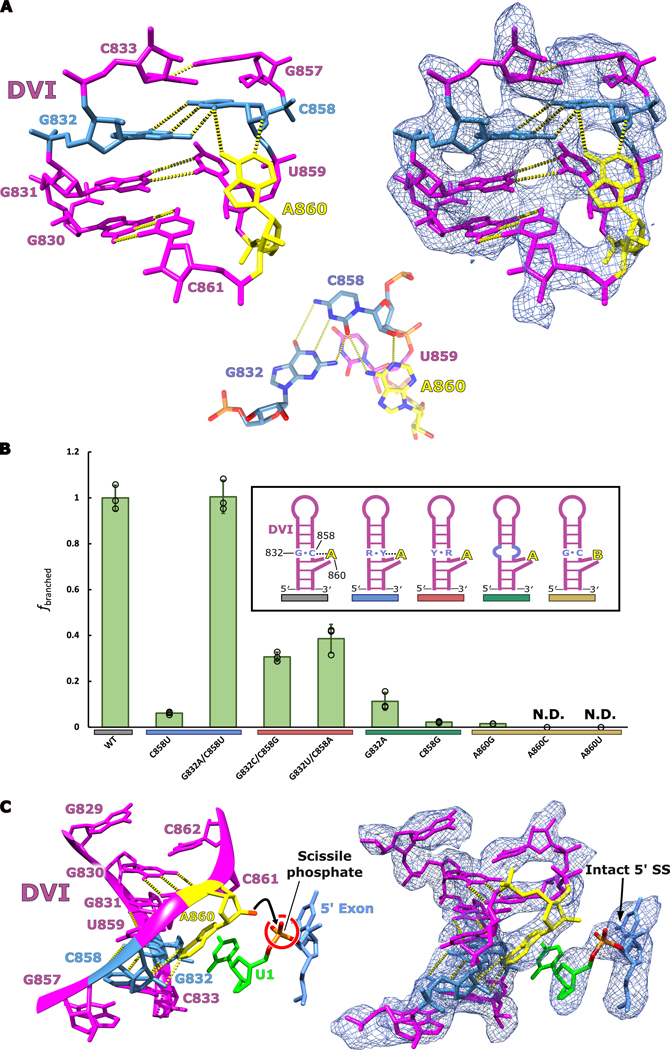
Fig 4. Active site architecture required for branching.
a. The branch-site adenosine (yellow) is held in position for the first step of splicing by a base triple formed between G832-C858-A860. An inset of the base triple is shown to highlight the geometry of the hydrogen bonding network. This model represents the conformation of DVI required for branching to occur at the 5′ SS. b. In vitro splicing assays were performed to investigate the importance the G832-C858-A860 base triple for branching. All mutants were tested in triplicate (n=3) and individual data points have been overlaid on the bar graph as a dot plot with error bars representing standard deviation. The fraction of branched normalized to WT activity is reported for all the mutants tested. Significant decreases in branching are observed if the base triple is disrupted through mutation. The G832A/C858U mutant displayed wild type branching activity suggesting that this mutation maintains the structural requirements to properly position the branch-site adenosine for nucleophilic attack. Simplified secondary structures of DVI that represent the intended effects of the mutations are shown. c. The branch helix adopts a conformation that ultimately extrudes the 2′-OH (red) of the branch-site adenosine (yellow) and points it directly towards the scissile phosphate (orange) of the 5′ SS. Intact density exists for the scissile phosphate of the 5′ SS supporting that this model represents the pre-branching state of splicing.