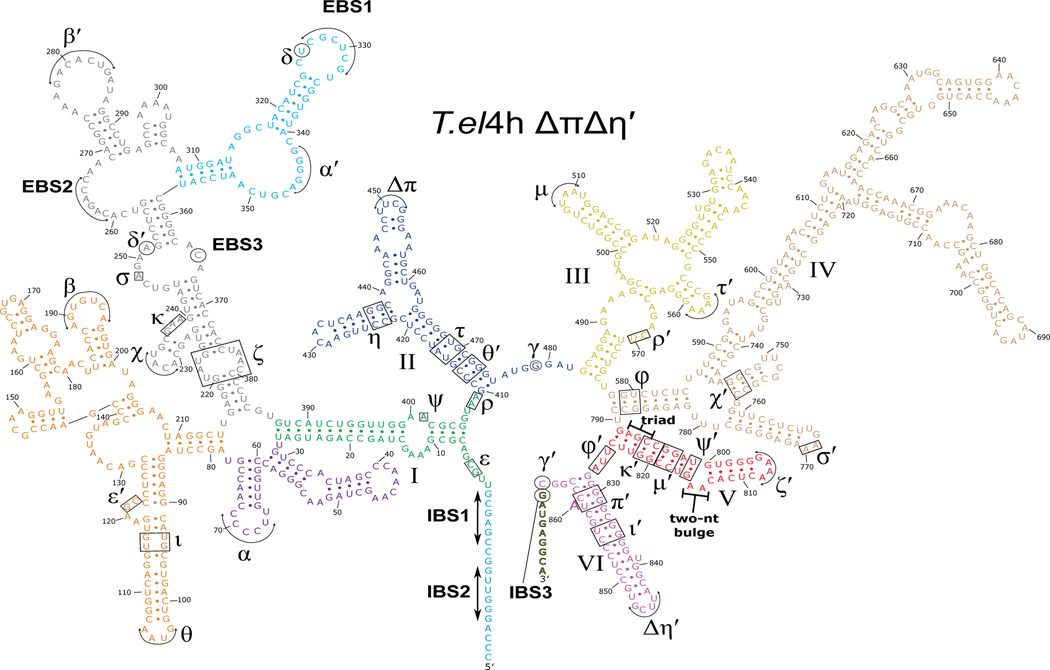
Extended Data Fig. 2. Secondary structure of T.el4h ΔπΔη′ group II intron.
The T.el4h ΔπΔη′ intron RNA is made up of six highly conserved domains labeled I-VI. Domain I contains several key tertiary interactions that act as a scaffold for the binding of the catalytic components. It also contains the exon binding sequences (EBS1, EBS2, and EBS3) that are responsible for base pairing the intron binding sites (IBS1, IBS2, and IBS3) within the exons to delineate the 5′ and 3′ splice sites. Domain II (blue) participates in two key tertiary interactions (π-π and η-η′) that help to control the branch helix dynamics involved in substrate exchange between steps of splicing. These two interactions are mutated from their native GNRA tetraloops to non-interactive UUCG tetraloops to allow the capture of pre-branching structural intermediates during subsequent cryo-EM experiments. Domain III (yellow) uses its tertiary interactions to help brace the intron and stabilize the active conformation. Domain IV (wheat) contains the open reading frame that encodes the maturase protein and provides the main binding platform for the maturase protein. Domain V (red) is the most highly conserved domain and harbors both the AGC triad and the two-nucleotide bulge that make up the active site of the intron. Domain VI (magenta), also known as the branch helix, contains the adenosine nucleophile (A860) used in the 1st step branching reaction to form the lariat bond with the 5′ SS.