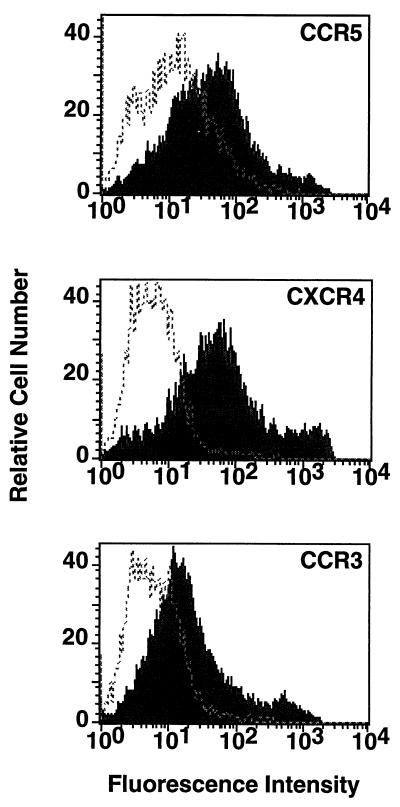
FIG. 1.
Surface expression of coreceptors. NIH 3T3 cells were transfected with pSC59-based plasmids containing a synthetic vaccinia virus promoter linked to the indicated coreceptor genes and coinfected with vCB-3 (CD4) and vCB21R-LacZ; control cells were transfected with the empty pSC59 plasmid and infected identically. Following overnight incubation to allow expression of vaccinia virus-encoded proteins, the cells were stained with the corresponding antibodies as follows: for CCR5, rabbit polyclonal antisera against a synthetic peptide representing the CCR5 extracellular N terminus (1:50 dilution) (4); for CXCR4, the 12G5 monoclonal antibody (23 μg/ml) (22), donated by J. Hoxie, University of Pennsylvania; and for CCR3, the 7B11 monoclonal antibody (20 μg/ml) (29), donated by C. Mackay, Leukosite. Detection was achieved with the following secondary antibodies (10 μg/ml; Boehringer Mannheim): for CCR5, fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G; and for CXCR4 and CCR3, fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G. The cells were washed, treated with 1 μg of ethidium bromide homodimer per ml, fixed with 0.1 ml of 4% paraformaldehyde, and analyzed with a FACSCAN flow cytometer (Becton Dickinson, Menlo Park, Calif.). Analyses of forward and side scatter as well as ethidium bromide homodimer fluorescence indicated nearly homogeneous populations of viable cells. Shaded profiles indicate coreceptor-expressing cells; unshaded profiles indicate control cells transfected with the empty pSC59 plasmid. The mean fluorescence intensities with coreceptor-expressing versus control cells were as follows: CCR5, 92 versus 21; CXCR4, 167 versus 4; and CCR3, 57 versus 9. In separate experiments, similar distinctions were seen when coreceptor-expressing cells were stained with either an anticoreceptor monoclonal antibody or an isotype-matched control monoclonal antibody (data not shown).