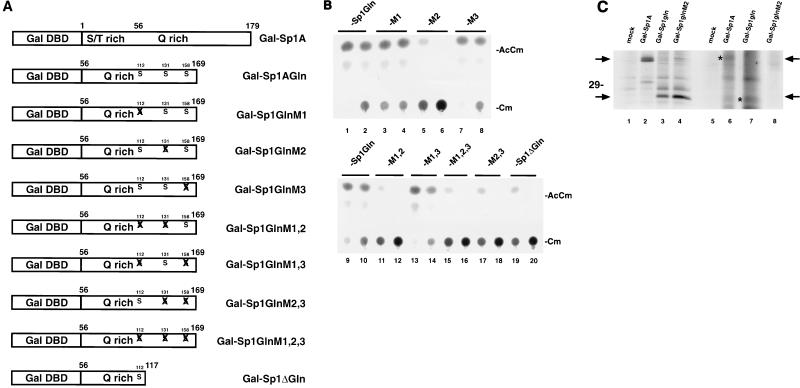
FIG. 8.
A point mutation at serine 131 in Sp1 reduces Tat transactivation. (A) Schematic representations of point mutations. Gal-Sp1A contains the N-terminal A domain of Sp1 (from amino acids 1 to 169), while Gal-Sp1Gln has the N-terminal serine/threonine-rich portion (amino acids 1 to 55) deleted. Relative locations of point mutations are indicated by X. M1, serine 112 to alanine; M2, serine 131 to alanine; M3, serine 158 to alanine. Combinations are indicated as M1,2, M1,3, etc. Gal-Sp1ΔGln is a C-terminal deletion (amino acids 118 to 169 removed) of Gal-Sp1Gln; serines 131 and 158 are absent from this construct. The amino acid position assignments are relative to the Sp1 sequence in GenBank (accession no. J03133). All mutations were verified directly by sequencing (data not shown). DBD, DNA-binding domain. (B) CAT assays of the various Gal-Sp1Gln mutant individually tested in the presence of coexpressed Tat protein. HeLa cells were transfected with Gal-HIV-1 LTR-CAT reporter, pSV-Tat expression plasmid, and one of the Gal-Sp1Gln mutants (-M1, -M2, or -M3). Each set of transfections used either 5 (left) or 1 (right) μg of Gal-Sp1 mutant plasmid. With the lower activity level of Sp1Gln arbitrarily set as 1, the comparable average relative activities from the mutants are as follows: M1, 1.3; M2, 0.05; M3, 1.1; M1,2, 0.02; M1,3, 1.5; M1,2,3, 0.02; M2,3, 0.1; and Sp1ΔGln, 0.1. AcCm, acetylated chloramphenicol; Cm, chloramphenicol. (C) Immunoprecipitation of Gal-Sp1 proteins. HeLa cells transfected with the indicated plasmids were labeled overnight with [35S]methionine-cysteine (lanes 1 to 4) or 32Pi (lanes 5 to 8). The cells were lysed in RIPA buffer and immunoprecipitated with antiserum raised to the Gal DNA-binding domain. The immunoprecipitates were resolved by SDS-PAGE (14% gel) followed by autoradiography. Arrows and asterisks indicate the positions of relevant bands. Note the absence of 32P-labeled immunoprecipitated band in Gal-Sp1GlnM2 (compare lanes 4 and 8).