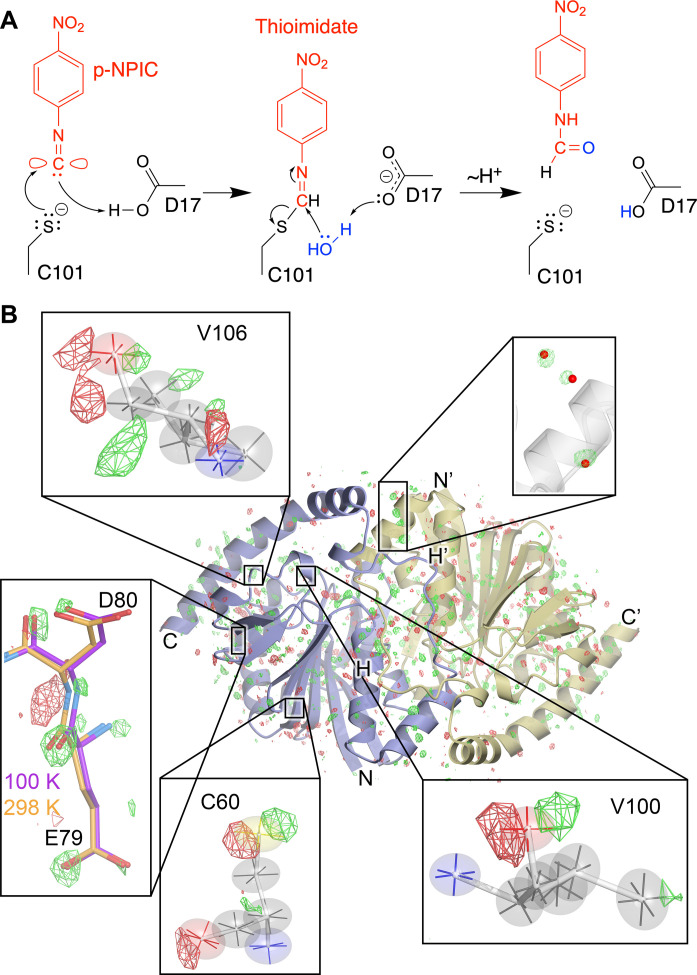
Fig. 1. ICH mechanism and evidence for temperature-dependent modes of intrinsic enzyme flexibility.
(A) Postulated ICH mechanism is shown for the para-nitrophenyl isocyanide (p-NPIC) substrate used in this study. The isocyanide is shown in its carbenoid form, consistent with the electrophilic character needed for attack by the catalytic Cys101 thiolate nucleophile in ICH. Formation of the thioimidate intermediate is postulated to be facilitated by the general acid Asp17, which protonates the C1 atom and then later acts as a general base to activate water (blue) for thioimidate hydrolysis. (B) Temperature-dependent changes in the ICH dimer. The Fo(274 K synchrotron)-Fo(298 K XFEL) isomorphous difference electron density map is contoured at 3σ, with positive features shown in green and negative features in red. Insets show regions of special interest, including the alignment of difference electron density features along the principal axes of anisotropic ADPs in Cys60, Val100, and Val106. Fo(274 K synchrotron)-Fo(298 K XFEL) peaks near ordered waters (red spheres, top) indicate higher occupancy of these waters at 274 K than 298 K. For residues 79 and 80, the difference electron density indicates displacements that agree with the structural differences observed in a 100 K crystal structure (purple bonds) compared to the 298 K XFEL structure (gold bonds).