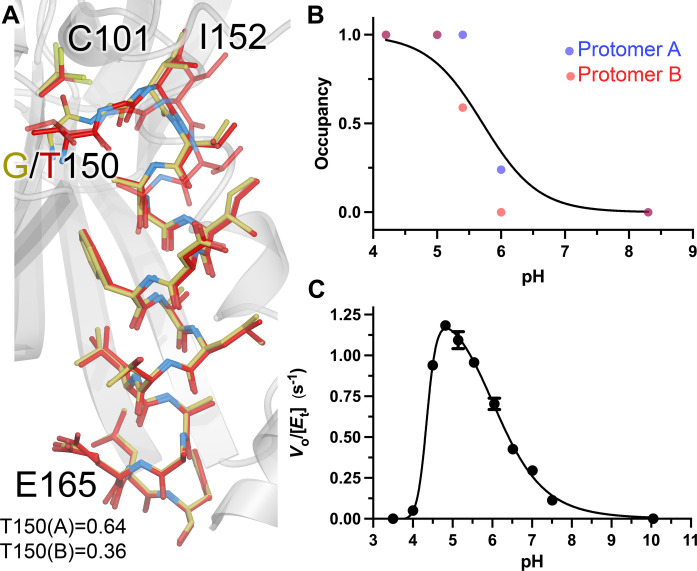
Fig. 2. G150T ICH predominantly populates conformations that are sampled by wild-type ICH upon Cys101 charge neutralization.
(A) Close structural agreement between the shifted conformation of helix H in resting G150T at pH 8.8 (red) and wild-type ICH at pH 4.2 (gold). Alternate conformations are shown as semi-transparent bonds, and occupancies are shown at the bottom. (B) Helix H conformational changes owing to Cys101 protonation. The refined occupancies of the shifted (i.e., G150T-like) conformation of helix H in crystal structures of wild-type ICH are plotted against pH. ICH crystallizes with two molecules in the ASU, with occupancies of helix H for protomer A shown as blue points and protomer B as red. The data are fitted to the Henderson-Hasselbalch equation (black; see Methods) with an apparent pKa of 5.7. (C) pH versus rate profile for wild-type ICH. Data were measured in triplicate, with error bars showing SEM and fitted using a dose-response curve with a rising inflection point at 4.4, maximum at 4.8, and falling inflection point of 6.1.