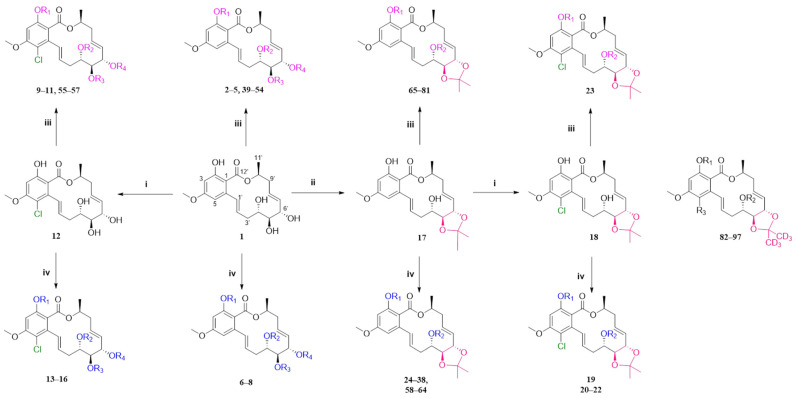
Scheme 1.
The synthetic route. i SO2Cl2, 0 °C; ii p-TsOH, acetone, room temperature, 5 h; iii ArCH2Br reagent, K2CO3, acetone, 50 °C, 24 h; iv anhydride, acyl chloride reagents or carboxylic acids, DMAP, EDCl, DCM, 45 °C, 3–4 h. Compounds 82–97 were synthesized in the same way as other compounds were, except acetone-d6 was used for acetal formation. The specific information of compounds 2–18, 20–23, 39–97 was shown in Ref. [27].