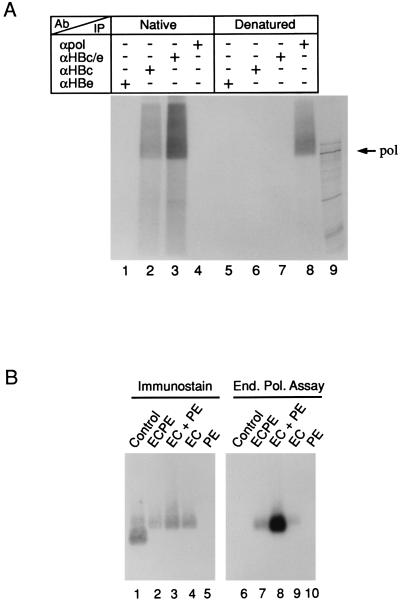
FIG. 5.
Pol activity is confined to the interior of the core particle. (A) Core particles from EC+PE-infected insect cells were purified by ultracentrifugation and then subjected to in vitro EPAs as described in Materials and Methods. For SDS-PAGE analysis of the covalently linked Pol-DNA complexes, reaction products were radioimmunoprecipitated under native (lanes 1 to 4) or denaturing (lanes 5 to 8) conditions using the following antibodies: HBe-specific MAb 2221 (lanes 1 and 5), HBc-specific MAb 3120 (lanes 2 and 6), rabbit anti-HBe/c-specific antiserum (lanes 3 and 7), and rabbit anti-Pol antiserum (lanes 4 and 8). See the text for further details. For visualization, the gel was dried and exposed to X-ray film. The migration of the ∼93-kDa in vitro-translated HBV Pol protein (lane 9) is indicated on the right. (B) Colocalization of radiolabeled in vitro Pol products and immunostained HBV nucleocapsids. Core particles were harvested from insect cell lysates ∼90 h postinfection with the ECPE (lanes 2 and 6), EC+PE (lanes 3 and 8), EC (lanes 4 and 9), and PE (lanes 5 and 10) recombinant baculoviruses as indicated above and then partially purified by ultracentrifugation. Aliquots were subjected to an in vitro EPA (End. Pol. Assay) and then resolved on a native 1% agarose gel. Following transfer to a nitrocellulose membrane, core proteins were immunostained with anti-HBe/c antiserum (lanes 1 to 5) and then exposed to X-ray film to visualize the 32P-labeled products (lanes 6 to 10). Core particles expressed in Escherichia coli (lanes 1 and 6) served as a reference.