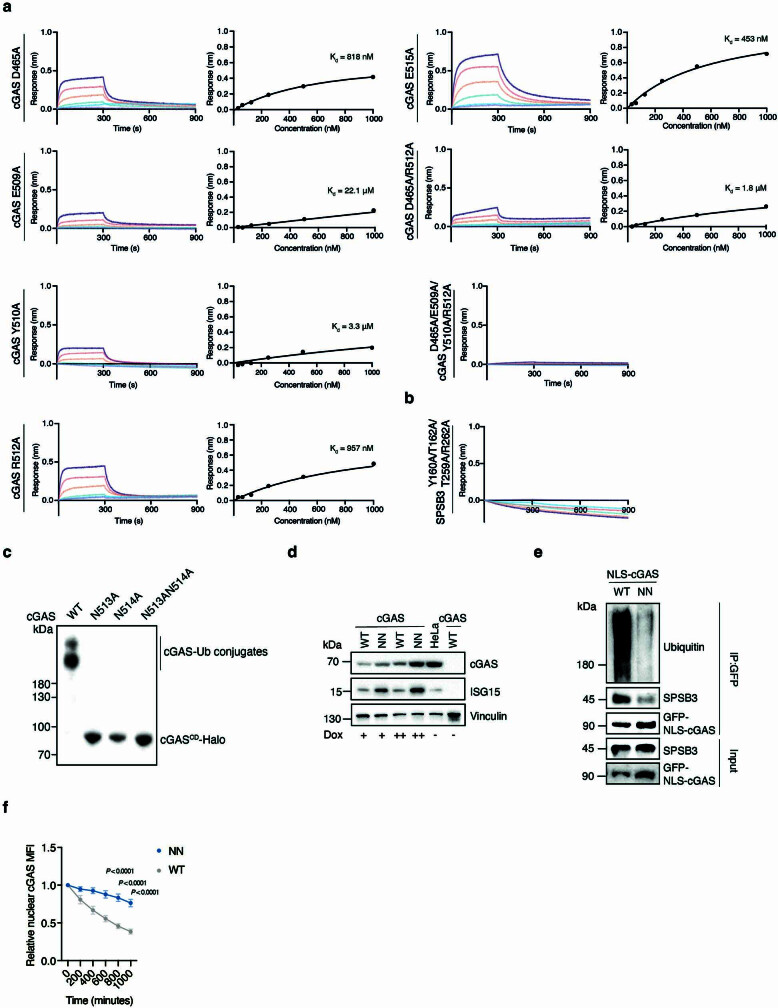
Extended Data Fig. 9. Effect of structure-guided mutations on cGAS binding to SPSB3.
a, Left panels: bio-layer interferometry binding assays of His-Halo-tagged cGAS mutants with SPSB3-ELOBC. Right panels: binding affinity of cGAS mutants with SPSB3-ELOBC. b, Bio-layer interferometry binding assays His-Halo-tagged cGAS with an SPSB3 mutant in complex with ELOBC. c, Immunoblot showing in vitro ubiquitylation reactions of wild-type human cGAS catalytic domain (CD; aa: 155–522) or distinct cGAS CD mutants by CRL5SPSB3. d, Cell lysates from HeLa cells, HeLa cGAS KO cells reconstituted with doxycycline-inducible wild-type cGAS (WT), cGAS(NN) treated with different concentrations of doxycycline for 3 days were analysed by immunoblot. Vinculin was used as a loading control. e, IP of GFP-tagged cGAS from HEK293T cells transfected with constructs for GFP-tagged NLS-cGAS (WT) or GFP-tagged cGAS(NN), HA-tagged ubiquitin, and SPSB3. Samples were analysed by immunoblot. f, Relative nuclear cGAS-GFP MFI measurement in post-mitotic HeLa cGAS KO cells reconstituted with cGAS (WT) or cGAS(NN) (n = 15 cells per condition). Data are mean ± SD. Two-way ANOVA with Šídák’s multiple comparison test was used for statistical analysis. One representative of two (d-e) or three (a-c) independent experiments is shown.