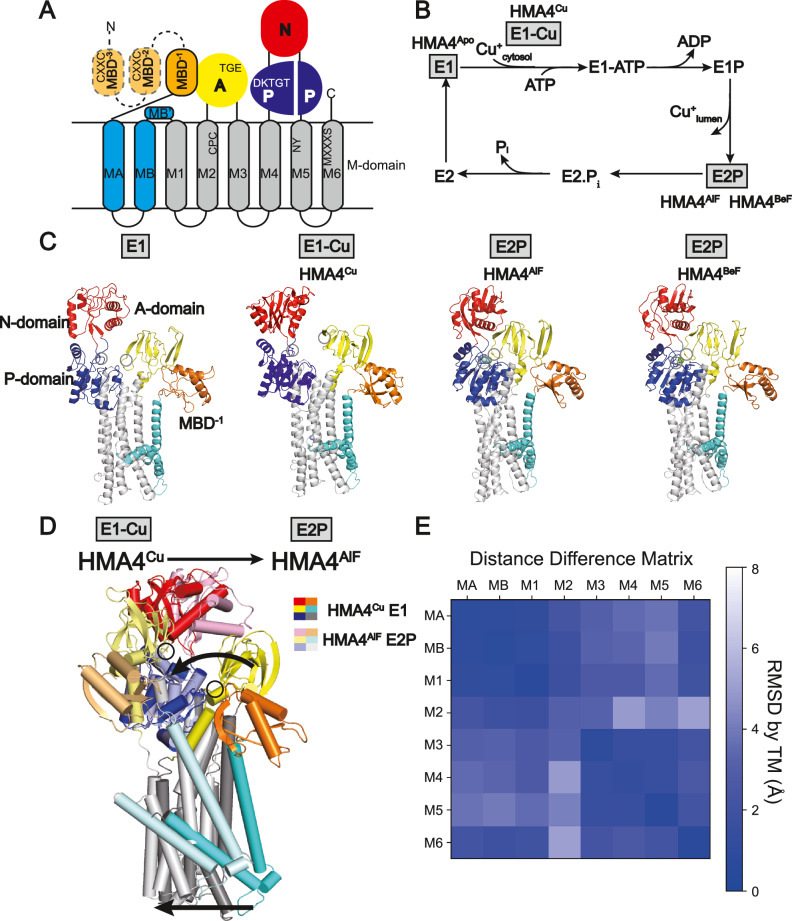
Fig. 1. Topology and E1-E2P-E2P-E2 Post-Albers transport cycle of copper transporting P-type ATPases, as well as structures of the HMA4 member from rice.
A Topology with the A- (actuator, yellow), P- (phosphorylation, blue), N- (nucleotide binding, red) and M-domains (transmembrane domain, cyan and gray), and 0–6 metal binding domains (MBDs, orange, 3 in HMA4). B Schematic transport mechanism with inward- (E1) and outward-facing (E2) states that undergo auto(de)phosphorylation to E1P and from E2P, respectively. The structurally determined conformations are highlighted in gray. C Cartoon representation of the cryo-EM structures of the HMA4 intermediates determined in this work, colored as in panel A. The TGE and DKTGT-motifs are shown with black circles. D Structural changes associated with the shift from the E1-Cu (HMA4Cu) to the E2P (HMA4AlF) state, colored as in panel A and in lighter shades, respectively. The circles represent the TGE-motif sites of the A-domain. Arrows highlight the changes. E Distance difference matrix in-between the E1 (HMAapo) and E2P (HMAAlF) states, showing the relative movement of each TM helix in the M-domain. The matrix was calculated as in84. MA-M1 and M3-M5 form separate rigid bodies. M2 moves considerably compared to M3-M6.