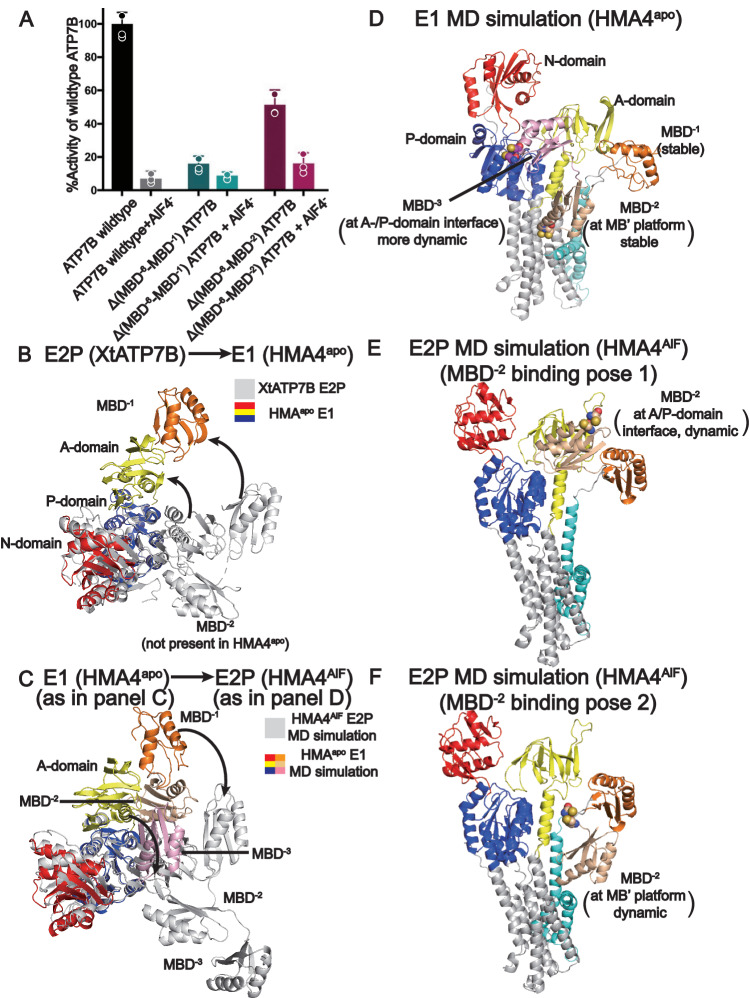
Fig. 3. The diverse functional roles of the metal binding domains (MBDs).
A Functional data comparing wildtype human ATP7B and truncations with no MBD (Δ(MBD−6-MBD−1)ATP7B) or only MBD−1 (Δ(MBD−6-MBD−1)ATP7B) attached to the ATPase core. Assessed through detection of released inorganic phosphate in detergent solution as triggered by copper and ATP in triplicate (n = 3 distinct biochemical samples). Data are presented as averages with standard deviation error bars. See also Supplementary Fig. 2. B Structural comparison of the cytoplasmic domains of the E2P (XtATP7B) and E1 states (HMA4apo) suggests the E2P → E1 transition can occur without MBD−2 interference. View from the cytoplasm. C Structural comparison of the E1 (as shown in panel D) and E2P states (as shown in panel E) suggests the E1 → E2P transition cannot occur without interference of MBD−3 when located at the suggested position. View from the cytoplasm. D MD simulations of the E1 state (HMBAapo) indicates MBD−2 is stable at the MB’ platform, while the MBD preceding MBD−2, MBD−3, is flexible, but can interact with the ATPase core at the A-/P-domain interface. Cysteines of the CXXC motifs shown as spheres. See also Supplementary Fig. 19. E, F MD simulations of the E2P state (HMBAAlF) imply MBD−2 is dynamic and can interact with the ATPase core at the A-/P-domain interface (panel D) and at the MB’ platform (panel E). Cysteines of the CXXC motifs shown as spheres in both panels. See also Supplementary Fig. 19.