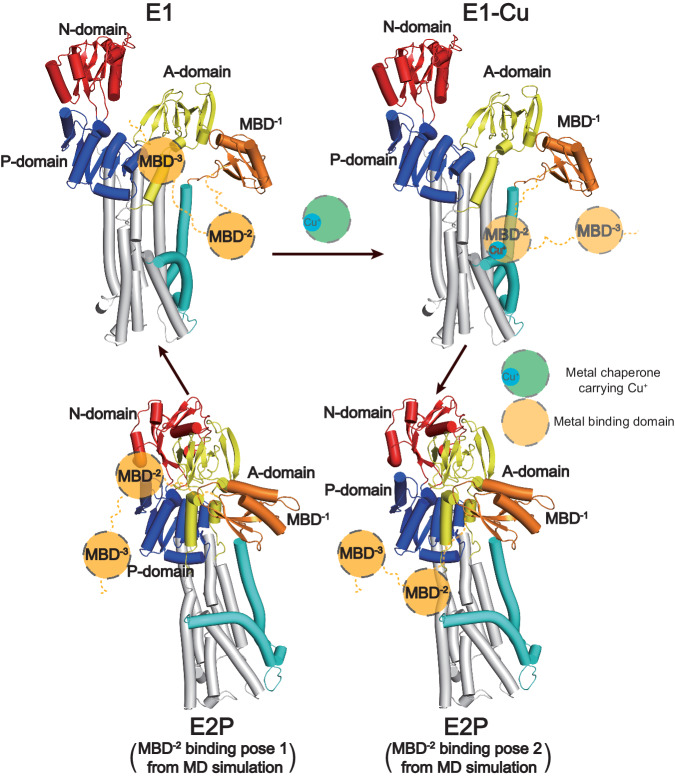
Fig. 5. Proposed transport and regulation mechanism of copper-specific P1B-ATPases.
MBD−1 is maintained linked to the A-domain throughout the E1-E1P-E2P-E2 transport cycle. The E1 state is inward-open and capable of binding MBD−3. At low copper concentrations, the state can likely be inhibited by MBD−3 through prevention of turn-over of the soluble domains. At elevated copper level, MBD−3 releases, copper is donated from soluble chaperones to MBD−2, and MBD−2 docks to the MB’ platform for ion delivery. This allows transfer of the metal to the conserved Met of M1 at the MB’ platform, and then to the cysteines of the CPC-motif (M4), thereby forming the entry site as observed in the E1-Cu intermediate. Conformational changes from E1-Cu to E1P establishes the high-affinity binding site consistent with the cysteines of the CPC-motif and the Met of the MXXXS-motif. This triggers accomplishment of the E2P form in which metal is shuttled through a pathway lined by MA, M2, and M6 via sulfur-exposing residues. MBD−2 can bind to this conformation but likely without inhibitory effect. Following copper release, the protein reverts to the E2 conformation from which a new cycle can be commenced.