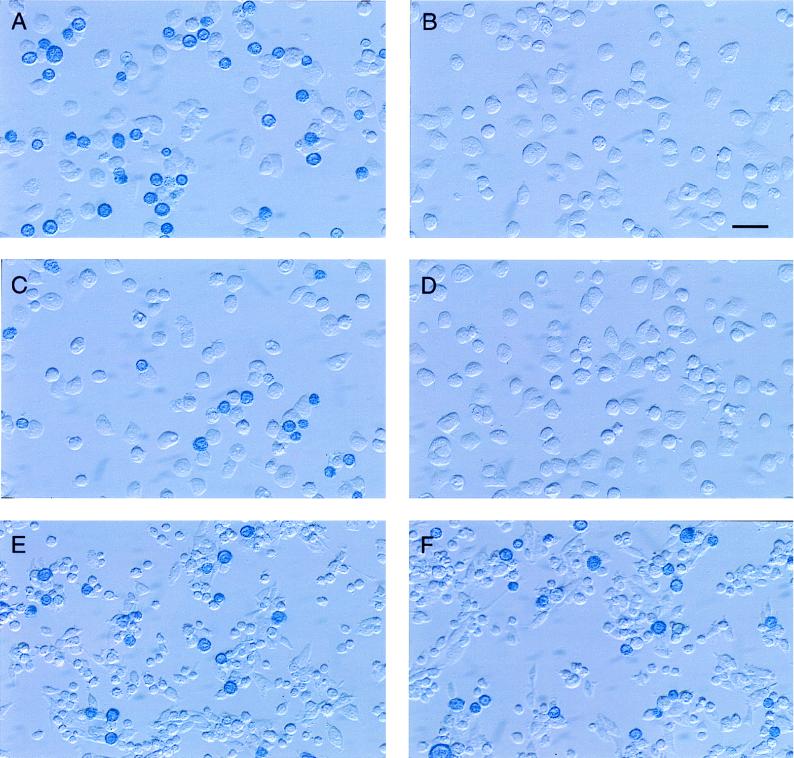
FIG. 5.
Assays of binding of H proteins from Montefiore 89 and Edmonston measles virus to mouse OST-7 cells expressing CD46 and marmoset B95-8 cells. H proteins from the Montefiore 89 and Edmonston strains of measles virus were cloned and expressed on the surface of Sf9 insect cells by using the recombinant baculovirus system. Sf9 cells also expressed β-galactosidase and were stained blue by the addition of Bluogal substrate. The blue insect cells were incubated with mouse cells expressing human CD46 (A and B), mouse cells expressing marmoset/human chimeric CD46 (C and D), or B95-8 cells (E and F), and loosely adsorbed Sf9 cells were washed away. Insect cells which expressed the Edmonston H protein remained attached to mouse cells expressing human or chimeric CD46 as well as to the marmoset B95-8 cell line (A, C, and E). The wild-type Montefiore H protein did not bind to mouse cells expressing human CD46 or chimeric CD46, but it did adhere to the marmoset B cell line (B, D, and F). Bar, 2.5 μm.