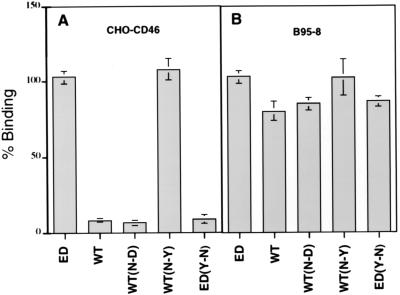
FIG. 7.
The Tyr481Asn mutation inhibits the binding of Edmonston and Montefiore 89 H proteins to CD46. Edmonston H (ED), Montefiore H (WT), and mutated forms of these proteins were expressed in Sf9 insect cells and incubated with CHO cells containing human CD46 (A) or marmoset B95-8 (B) cells. As expected, insect cells expressing Edmonston H (ED) bound to both CHO-CD46 and B95-8 cells, while wild-type Montefiore 89 H (WT) protein bound only to B95-8 cells. The N416D mutation introduced into Montefiore 89 H [WT(N-D)] had no effect on binding to either cell line. However, an N481Y mutation in the wild-type H [WT(N-Y)] converted the protein to a CD46-binding phenotype. In addition, when the Y481N mutation was placed in Edmonston H [ED(Y-N)], binding to CHO-CD46 cells was abolished. None of the mutations affected the binding of either Edmonston or wild-type H proteins to marmoset B95-8 cells. Binding was measured by quantitating β-galactosidase activity and was expressed as a percentage relative to the binding observed for Edmonston H protein.