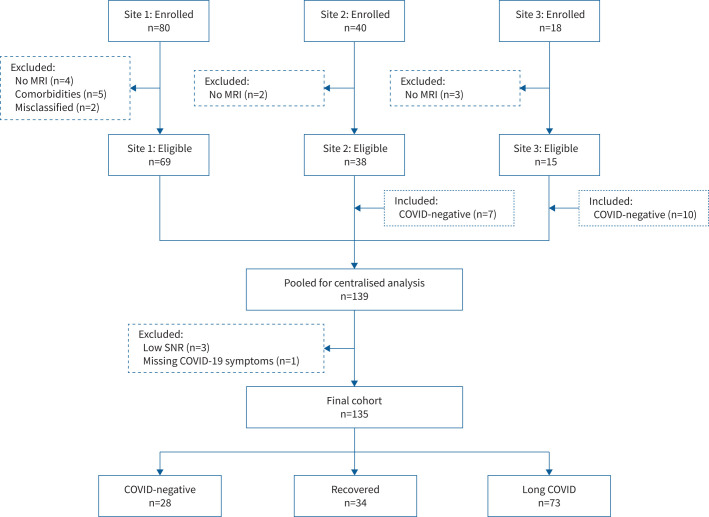
FIGURE 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram of enrolment of participants. Of 80 participants enrolled at Site 1, nine were excluded (four did not complete magnetic resonance imaging (MRI) and five were COVID-negative or fully recovered with comorbidities or exposures that could influence 129Xe MRI (severe COPD (n=2), rheumatoid arthritis (n=1), HIV (n=1), and heavy current daily cannabis and tobacco smoking (n=1))) and two were deemed misclassified (no positive coronavirus disease 2019 (COVID-19) test and negative antibody test (n=1), and positive COVID-19 test between pulmonary function tests and MRI (n=1)). Site 2 and Site 3 enrolled 40 and 18 participants, respectively, of which two at Site 2 and three at Site 3 did not complete MRI and were excluded. Site 2 (n=7) and Site 3 (n=10) retrospectively included COVID-negative controls, whereas Site 1 prospectively enrolled. From 139 participants pooled for centralised analysis, four were further excluded (low 129Xe gas signal-to-noise ratio (SNR) (n=3) and missing COVID-19 symptoms for classification as recovered or long COVID (n=1)) and the final study group consisted of 135 participants in total (n=28 COVID-negative, n=34 fully recovered and n=73 long COVID).