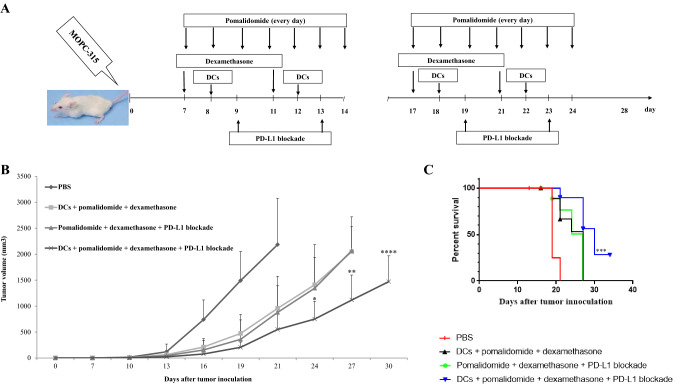
Fig. 2.
In vivo animal vaccination. Four treatment groups were established: (1) PBS control, (2) tumor antigen-loaded dendritic cell (DC) vaccination + pomalidomide with dexamethasone, (3) pomalidomide with dexamethasone + programmed death-ligand 1 (PD-L1) blockade, and (4) DCs + pomalidomide with dexamethasone + PD-L1 blockade. a Schematic representation of the combination of DCs + pomalidomide with dexamethasone + PD-L1 blockade. On day 0, mice were injected subcutaneously with 5 × 105 MOPC-315 cells in a volume of 0.1 mL into the right flank. After tumor growth, pomalidomide (0.06 mg/kg/day) was orally administered once a day for 18 days with a 3-day break after the first 8-day dosing period, and dexamethasone (0.6 mg/kg/day) was injected intravenously in a volume of 0.1 mL on days 7, 11, 18, and 21. PD-L1 blockade (200 µg/mouse) was injected intraperitoneally in a 0.1-mL volume on days 9, 13, 19, and 23. Each dose of DCs (1 × 106 per mouse) was injected subcutaneously into the left flank of BALB/c mice in a volume of 0.1 mL PBS on days 8, 12, 18, and 22. b Data are presented as mean ± standard error of the mean (SEM) and are representative of two independent experiments. c The survival of the tumor-bearing mice is shown. The combination of DCs + pomalidomide with dexamethasone + PD-L1 blockade significantly inhibited tumor growth (*, P < 0.05 on day 24) and induced a long-term systemic anti-myeloma immune response (34 days)