Abstract
Background: Digital health applications (DiGA) supporting the management of diabetes are among the most commonly available digital health technologies. However, transparent quality assurance of DiGA and clinical proof of a positive healthcare effect is often missing, which creates skepticism of some stakeholders regarding the usage and reimbursement of these applications. Methods: This article reviews the recently established fast-track integration of DiGA in the German reimbursement market, with emphasis on the current impact for manufacturers, healthcare providers, and people with diabetes. The German DiGA fast track is contextualised with corresponding initiatives in Europe. Results: The option of a provisional prescription and reimbursement of DiGA while proving a positive healthcare effect in parallel may expedite the adoption of DiGA in Germany and beyond. However, hurdles for a permanent prescription and reimbursement of DiGA are high and only one of 12 that have achieved this status specifically addresses people with diabetes. Conclusion: The DiGA fast track needs to be further enhanced to cope with remaining skepticism and contribute even more to a value-based diabetes care.
Keywords: clinical trials, digital health, evidence, Germany, reimbursement, value-based healthcare
Introduction
Diabetes can be considered a data management disease and a paradigm example of how the real-world acquisition of health and treatment data informs an integrated and personalized chronic disease management. 1 For both patients and diabetes care providers, it is a piece of normality already today to use continuously collected metabolic, treatment, and lifestyle data in the process of joint decision-making on treatment. Therefore, it is not surprising that digital health technologies (DHT)—including digital health applications (“apps”) proposed to support diabetes self-management—are among those most commonly available. 2 The vast range of apps in the diabetes field supports the adoption of lifestyle interventions for the prevention and management of diabetes, the evaluation of blood glucose control quality, and the dosing of insulin. 2
Most apps realize a “Software-as-a-Service” (SaaS) model with a mode of action and effect sizes that may differ from those of drugs and conventional medical products. Many apps are not well integrated into workflows of care provision. Since app developers (often startup companies) have little expertise in clinical research and the implementation of clinical trials is considered a time- and cost-intensive challenge, evidence of positive healthcare effects is often still pending. Accordingly, there is a shortage of quality-assured apps that can be reimbursed by healthcare insurance funds. 3 Initiatives like the DiaDigital by the German Working Group for Diabetes Technology offered an evaluation of apps on a voluntary basis, involving healthcare providers and patients. 3 However, the outcome did not confirm any added value compared to therapies that are already prescribable.
On the Fast Track to Reimbursement: The German Directory of Digital Health Applications
The German Digital Healthcare Act (DVG) was enacted in 2019 aiming at increasing the adoption in the German healthcare system of high-quality DHT from all over the world across all disease indications. 4 The corresponding ordinance (DiGAV) established a fast track pathway for digital health applications (in German, DiGA) to be reimbursed by statutory health insurances (“apps on prescription”). 5 Curated by the German Federal Institute for Drugs and Medical Devices (BfArM), the fast track enables the prescription of DiGA to more than 73 million people insured under the German statutory health insurance scheme. 6
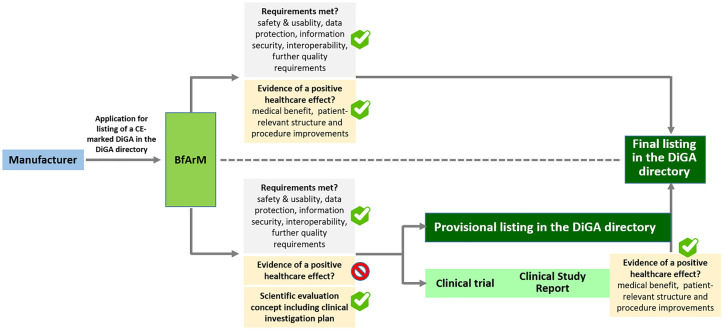
DiGA, which can enter the fast track, are class I or IIa medical devices for the treatment (not primary prevention) of a disease, which main functionalities are based on digital technology. They should be mainly aimed at patients and not the healthcare team, and they are expected to help patients to improve their disease management skills (Table 1). 6 DiGA can be prescribed and are eligible for full reimbursement if listed either provisionally or permanently in the DiGA directory maintained by the BfArM. 6 Although both provisionally and permanently listed DiGA must comply with the European Medical Device Regulation (EU MDR 2017/745) and meet high data protection, information security, and interoperability standards, a permanent listing also requires the demonstration of scientifically proven evidence of a positive healthcare effect (Figure 1). 7
Table 1.
Classification of Positive Care Effects of DiGA the Proof of Which Is a Prerequisite for the Reimbursement of Costs by the Statutory Heath Insurers in Germany.
| Positive care effects | |
|---|---|
| Medical benefits | Patient-relevant structure and procedure improvements |
| Improving health status | More effective coordination of treatment procedures |
| Shortening the duration of the disease | Closer alignment of treatments with guidelines and recognized standards |
| Prolongation of survival | Improved treatment adherence |
| Improved quality of life | Easier access to care |
| Improved patient safety | |
| Better health literacy | |
| Patient sovereignty | |
| Better coping with illness-related everyday problems | |
| Reduction of therapy-related efforts and burdens for patients and their relatives | |
A special focus is on the patient-centering of the positive effects that must be demonstrated to the Federal Institute for Drugs and Medical Devices (BfArM) by the manufacturer. The workload of medical professionals or health or health economic indicators are not classified as patient-relevant endpoints in this context. 6
Abbreviation: DiGA, Digital Health Applications.
Figure 1.
The German fast-track procedure of a listing in the DiGA directory. Manufacturers apply for listing of their DiGA in the DiGA directory by submitting the package of required documents to the BfArM. In case all requirements including the demonstration of evidence of a positive healthcare effect are met, BfArM will grant a final listing of the DiGA in the DiGA directory within three months of receipt of the documents. In case all requirements are met, except from demonstration of evidence of a positive healthcare effect, BfArM can grant—also within a period of three months—a provisional listing of the DiGA in the DiGA directory. However, to receive a provisional listing, the manufacturer has to present a scientific evaluation concept including a clinical investigation plan prepared by an institution independent of the manufacturer. Manufacturers of provisionally listed devices need to provide evidence of a positive healthcare effect within a period of 12 months from the granting of the provisional listing. The timeframe can be extended once for another period of 12 months. Based on the trial outcomes documented in a clinical study report, BfArM evaluates the presented evidence of a positive healthcare effect within a period of three months. In the event of a positive evaluation result, BfArM approves the final listing of the DiGA in the DiGA directory. Through both the final and the provisional listing in the DiGA directory, the DiGA is covered by statutory health insurance nationwide and can be prescribed by any doctor or psychotherapist. Following the final listing in the DiGA directory, the manufacturer negotiates the amount of remuneration with the National Association of Statutory Health Insurance Funds (GKV-SV), which then replaces the previously applicable price set by the manufacturer. Abbreviations: DiGA, Digital health applications; BfArM, Federal Institute for Drugs and Medical Devices.
As a prerequisite for a provisional admission into the directory of DiGA, the DiGA manufacturer presents a scientific evaluation concept for a clinical trial that must be created by an independent scientific institute and meet the BfArM’s quality requirements for demonstrating evidence of a positive healthcare effect. The ISO 14155 (good clinical practice)-compliant clinical trial should be carried out in the intended patient population and care settings and the DiGA must be compared against existing standards of care, nontreatment, or to a DiGA already permanently listed. 5 Within usually one year, a formal clinical study report should be submitted by the manufacturer to BfArM 6 (Figure 1). The real-world experience gained during this timeframe can inform the further enhancement of the DiGA and the design of future clinical trials. BfArM grants a permanent listing of the DiGA in the directory if sufficient scientific evidence of a positive healthcare effect is demonstrated along the lines of patient-reported outcome measures as well as endpoints related to medical outcomes and healthcare experience.
Fast Track Experience so Far
Currently, as of August 9, 2022, only 12 out of 33 DiGA are permanently listed in the DiGA directory. 8 Among the permanently listed, HelloBetter solely aims at diabetes treatment and provides a 12-week online course for people with diabetes and depression, based on cognitive behavioral therapy strategies. 9 Only two other DiGA immediately targeting diabetes can be found within the 21 provisionally listed DiGA: The Esysta App & Portal and Vitadio. Esysta supports the management of people with diabetes on insulin therapy with an automatically kept diary that imports data from a smart insulin pen and a blood glucose meter. It also includes a web portal presenting continuously updated analyses of the data and facilitates communication between the patient and the diabetes team. 10 Vitadio uses a multi-modal learning therapy approach to support the self-management of people with type 2 diabetes including a personal advisor and a peer support group. 11 There are two other provisionally listed DiGA tackling metabolic diseases: Oviva and Zanadio. Both provide behavioral therapy support of multi-modal obesity therapy.12,13
The fact that only five DiGA targeting people with metabolic conditions are listed indicates that the directory may not yet fully serve all relevant disease indications. At the same time, it should be noted that the hurdles for inclusion in the directory are high: Of the 89 applications submitted by August 2021, only four were rejected, but 42 were initially withdrawn by manufacturers due to data analysis problems, not enough evidence or unsuitable endpoints. 14
Despite the high quality standards required by law, healthcare providers, health insurers, and manufacturers see room for improvement when it comes to the practical implementation of DiGA.15,16 BfArM evaluates DiGA mainly in the context of the preexisting service portfolio rendered by healthcare providers accredited by the statutory health insurances. 6 Accordingly, the prescription of DiGA, the training of patients in its compliant usage, the informed evaluation of the health data created by the DiGA, their integration in therapeutic decision-making, and required quality assurance processes are not yet adequately remunerated. As a result, many physicians are skeptical about DiGAs and prescription rates are lower than planned. So far, DiGA offer hardly alternatives that could replace existing therapy regimens. Most DiGA can be considered as complementary adjuncts to already established therapies. In practice, DiGA in diabetes care mainly complement the prescription of established drugs and diabetes technology. While automated insulin delivery systems have the potential to largely replace all open loop insulin therapy regimens, 17 they are currently excluded from the DiGA pathway due to their high-risk class classification according to the MDR 2017/745.
On the side of health insurance companies, the main criticism is that costs for DiGA are high—on average 400 euros per quartile—during the preliminary listing although prove of healthcare benefits are still pending. 16 On December 15, 2021, statutory health insurances (in German, GKV) and manufacturers’ associations have reached a compromise on pricing of DiGAs through arbitration. 18 According to the agreement, dynamics in pricing, thresholds, and exceptions will be used to determine the price of a DiGA. The expectation is that the price range will be between 200 and 250 euros and the first validity date is expected to be August 1, 2022. Apart from that, the integration of DiGA into healthcare processes orchestrated and monitored by healthcare professionals is not always ensured, leading to considerable dropout rates due to app fatigue or noncompliant use.15,19 From the point of view of DiGA manufacturers, (often startup companies) the preparation of the fast track application can be regarded as demanding and resource intensive. Conducting randomized controlled clinical trials (RCT) is considered a real challenge 20 : Performance of RCT is often hardly affordable and its outcomes do not always reflect effectiveness under conditions of real-world healthcare. 21 It requires high disease-specific and operational experience in clinical research to establish a meaningful control group (checking also usage of a mock DiGA as a placebo) to ensure the assignment of trial outcomes as accurate as possible to the DiGA under evaluation. 22 Although the performance of a RCT is not mandatory, it has been used by more than 90% of manufactures to evaluate a DiGA. 23
Other Fast Track–Related Initiatives in Europe
The National Institute for Health and Care Excellence (NICE) in the UK has developed a fine-grained evidence standard framework for DHT that differs from the DiGA pathway. 24 In the British framework, the risk-benefit ratio and the cost intensity of an individual DHT are closely linked to the level of evidence required to demonstrate eligibility for reimbursement. RCT conducted in a setting that is relevant to the U.K. health and social care system are considered best practice standard for the generation of high-quality evidence for effectiveness. Moreover, the presentation of a health economic analysis suitable to inform the pricing is mandatory for manufacturers. For cost-intensive DHT, a cost-utility analysis is the standard. Higher costs usually need to be justified by a corresponding gain in quality-adjusted life years. The NICE framework does neither provide for a provisional phase to prove a positive healthcare effect with full reimbursement at the same time nor exclude higher classified DHT from evaluation. In addition, separate standards apply to DHT utilizing artificial intelligence-powered adaptive algorithms.
Belgium has launched a platform (mHealthBELGIUM) for patients, healthcare professionals, and healthcare institutions, which assigns mobile apps to three different levels of a validation pyramid. 25 Apps are required to be compliant with the EU MDR2017/745 and the EU General Data Protection Regulation (level 1), to meet all criteria regarding data authentication, security, and interoperability as demonstrated by an independently conducted risk assessment (level 2), and to prove a social-economic benefit (level 3). Only apps that have reached the third level are eligible for reimbursement. So far, 13 apps for people with diabetes are presented: nine at the first level, as well as four at the second level. 26 On April 18, moveUP—used to support rehabilitation of patients with knee or hip prosthesis—was the first app to reach the eligibility for reimbursement (level 3). 27
According to press releases, French President Emmanuel Macron has announced to largely replicate the German fast track model in France. 28 To advance the internationalization of the fast track, pan-European public-private partnerships such as EIT Health are entitled to implement flagship projects involving multiple stakeholders to prove the effective usage of DiGA in different healthcare systems and inform the design of harmonized evaluation policies. 29
Learnings and Future Prospects
The German fast track is an internationally well-recognized path as it gives manufacturers the opportunity to achieve eligibility for reimbursement before a clinical trial can demonstrate that their DiGA achieve positive healthcare effects. It can lead to a triple win for manufactures, patients, and the German healthcare system. On the side of manufactures, it can facilitate the launch of their DiGA in the German market but also in other markets in Europe and beyond. For patients and healthcare providers, the DiGA directory provides transparency about quality-secured offers. At last but not least, the integration of global cutting-edge technologies in healthcare can speed up the implementation of a national e-health infrastructure. 30
In order to resolve the still widespread skepticism about DiGA, it could be helpful to take into account the needs of health insurers, healthcare professionals, and patients in the decision-making. The adequate payment of healthcare professionals for the provision of DiGA-related services that go beyond those previously agreed with the Statutory Health Insurance Fund will help to achieve a widely accepted and fully integrated usage of DiGA. Coupling pricing with the level of available evidence for a positive healthcare effect following the example of the NICE framework could help health insurers to manage their risk of financial misinvestment. This could be achieved by using adaptive licensing models, 31 or making performance-based managed entry agreements in the form of a patient-level payment-by-result or a population-level coverage basis. 32
Combining the risk-based approach of the EU MDR 2017/745 to clinical investigations with patient-reported outcome measures and health economic impact could pave the way for a fair pricing of DiGA in value-based healthcare settings. 33 An immediate reimbursement of cost-intensive devices could be granted for well-defined patient groups as long as high-level evidence proof is obtained through the performance of rigorously designed pre-market RCT accompanied by strong cost-utility assessments. DiGA that initially have a lower level of medical and/or health economic evidence would be entitled of a lower amount of reimbursement. Inspired by the US Pre-cert model, 34 continued post-market clinical follow-up activities including clinical investigations and the assessment of patient-reported outcome measures could inform a value-based adjustment of reimbursement levels.
For people with diabetes, a further development of the German fast track toward the inclusion of higher classes of DHT such as Automated Insulin Delivery systems 17 or cardiorespiratory health remote monitoring tools35,36 would be desirable. Rigorous RCT evaluating DHT have proven feasible when evaluating insulin dose optimization in youths with type 1 diabetes enabled by an automated artificial intelligence (AI)-based decision support system. 37 This level of rigorous evaluation is suitable to resolve skepticism and to create trust among various stakeholders regarding AI-powered DHT in healthcare.
Accordingly, fast track requirements on the scientific evaluation concept and the clinical study report should adopt the current extension of the SPIRIT-AI 38 and CONSORT-AI 39 guidelines regarding the use of DHT utilizing AI. Overall, an upgraded edition of the German DiGA pathway could advance flagship projects of integrated and personalized diabetes management as the recently proposed digital diabetes clinic. 40 Further streamlining of the fast track application will help to prevent manufacturers from striving for less quality assured approaches to target patients outside the reimbursement market. In this way, the German fast track pathway would become a greater role model with regard to the implementation of national e-health infrastructures. In Germany and elsewhere, an accelerated availability of high-quality DiGA and higher classified DHT can make a tremendous contribution to the sustainability of healthcare, taking the challenge of demographic change with an increasing incidence of diabetes and a simultaneous shortage of healthcare professionals.
Footnotes
Abbreviations: AI, Artificial Intelligence; app, application software; BfArM, Federal Institute for Drugs and Medical Devices; CONSORT-AI, Consolidated Standards of Reporting Trials–Artificial Intelligence; DHT, Digital Health Technology; DiGA, digital health application; DVG, digital healthcare act; EIT, European Institute for Innovation and Technology; e-health, electronic health; EU, European; FDA, Food and Drug Administration; GKV, statutory health insurances; ISO, International Organization for Standardization; MDR, Medical Device Regulation; NICE, National Institute for Health and Care Excellence; RCT, randomized controlled clinical trials; SaaS, software as a service; SPIRIT-AI, Standard Protocol Items: Recommendations for Interventional Trials–Artificial Intelligence.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: F.S. and T.A.D. are full-time employees of Profil Institut für Stoffwechselforschung GmbH. L.H. is a shareholder of Profil Institut für Stoffwechselforschung GmbH. F.S. is a member of the EIT Health Supervisory Board. J.-M.B. and N.G. are full-time employees of the EIT Health e.V. and the EIT Health Colocation Center Germany–Switzerland, respectively. F.S., T.A.-D., C.S., J.S., M.J., and D.M.-W. are part of the EIT Health-funded RealWorld4Clinic consortium.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article is part of the pan-European Horizon Europe 220642-RealWorld4Clinic project (https://eit-health.de/realworld4clinic/) which receives funding from EIT Health. EIT Health is supported by the European Institute of Innovation and Technology (EIT), a body of the European Union.
ORCID iDs: Freimut Schliess  https://orcid.org/0000-0002-8232-9971
https://orcid.org/0000-0002-8232-9971
Lutz Heinemann  https://orcid.org/0000-0003-2493-1304
https://orcid.org/0000-0003-2493-1304
References
- 1. Jones A, Bardram JE, Baekgaard P, et al. Integrated personalized diabetes management goes Europe: a multi-disciplinary approach to innovating type 2 diabetes care in Europe. Prim Care Diabetes. 2021;15(2):360-364. [DOI] [PubMed] [Google Scholar]
- 2. Fleming GA, Petrie JR, Bergenstal RM, Holl RW, Peters AL, Heinemann L. Diabetes digital app technology: benefits, challenges, and recommendations. A consensus report by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) diabetes technology working group. Diabetes Care. 2020;43(1):250-260. [DOI] [PubMed] [Google Scholar]
- 3. Kaltheuner M, Drossel D, Heinemann L. DiaDigital apps: evaluation of smartphone apps using a quality rating methodology for use by patients and diabetologists in Germany. J Diabetes Sci Technol. 2019;13(4):756-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bundesministerium für Gesundheit. Digitale-Versorgung-Gesetz-DVG. https://www.bundesgesundheitsministerium.de/en/digital-healthcare-act.html. Published November 7, 2019. Accessed June 3, 2022.
- 5. Bundesministerium für Gesundheit. Digitale-Gesundheitsanwendungen-Verordnung-DiGAV. https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Gesetze_und_Verordnungen/GuV/D/DiGAV_RefE.pdf. Published April 9, 2020. Accessed June 3, 2022.
- 6. Federal Institute for Drugs and Medical Devices (BfArM). The fast-track Process for Digital Health Applications (DiGA) according to Section 139e SGB V—a guide for manufactures, service providers and users. https://www.bfarm.de/SharedDocs/Downloads/EN/MedicalDevices/DiGA_Guide.html. Accessed August 24, 2022.
- 7. Lauer W, Lobker W, Hofgen B. Digital health applications (DiGA): assessment of reimbursability by means of the “DiGA Fast Track” procedure at the Federal Institute for Drugs and Medical Devices (BfArM). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2021;64(10):1232-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Federal Institute for Drugs and Medical Devices (BfArM). DiGA-verzeichnis. https://diga.bfarm.de/de/verzeichnis. Date Unknown. Accessed August 9, 2022.
- 9. Federal Institute for Drugs and Medical Devices (BfArM). DiGA verzeichnis—hellobetter diabetes und depression. https://diga.bfarm.de/de/verzeichnis/1376. Published November 12, 2021. Accessed August 9, 2022.
- 10. Federal Institute for Drugs and Medical Devices (BfArM). DiGA—verzeichnis—ESYSTA app & portal—digitales diabetesmanagement. https://diga.bfarm.de/de/verzeichnis/939. Published April 7, 2021. Accessed August 9, 2022.
- 11. Federal Institute for Drugs and Medical Devices (BfArM). DiGA- verzeichnis—vitadio. https://diga.bfarm.de/de/verzeichnis/746. Published April 15, 2022. Accessed August 9, 2022.
- 12. Federal Institute for Drugs and Medical Devices (BfArM). DiGA—verzeichnis—oviva direkt für adiposas. https://diga.bfarm.de/de/verzeichnis/872. Published March 10, 2021. Accessed August 9, 2022.
- 13. Federal Institute for Drugs and Medical Devices (BfArM). DiGA—verzeichnis—zanadio. https://diga.bfarm.de/de/verzeichnis/294. Published October 22, 2020. Accessed August 9, 2022.
- 14. Lauer W, Lobker W, Sudhop T, Broich K. Digital health applications (DiGA) as an innovative component in digital healthcare in Germany-information, experiences, and perspectives. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2021;64(10):1195-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deloitte. Ein Jahr DiGA chancen für gesetzliche krankenkassen. https://www2.deloitte.com/de/de/pages/life-sciences-and-healthcare/articles/digitale-gesundheitsanwendungen-diga-studie.html. Published 2021. Accessed June 3, 2022.
- 16. GKV-Spitzenverbandes. Positionspapier des GKV- Spitzenverbandes: anforderungen und kriterien an digitale gesundheitsanwerdungen. https://www.gkv-spitzenverband.de/media/dokumente/service_1/publikationen/Positionspapier_DiGA_2021-01-07_barrierefrei.pdf Published 2020. Accessed August 24, 2022.
- 17. Schliess F, Heise T, Benesch C, et al. Artificial pancreas systems for people with type 2 diabetes: conception and design of the European CLOSE project. J Diabetes Sci Technol. 2019;13(2):261-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bundesschiedssämter. Schiedsstelle to § 134 Abs. 3 SGB V. https://schiedsstelle.de/media/dokumente/schiedsstellen/134/Rahmenvereinbarung_nach__134_Abs._4_und_5_SGB_V_Stand_16.12.21.pdf Date unknown. Accessed August 24, 2022.
- 19. Kulzer B, Heinemann L. D.U.T.—Digitalisierungs—und Technologiereport Diabetes. https://www.dut-report.de/digitalisierungsreport-2022/. Published 2022. Accessed June 3, 2022.
- 20. Stern AD, Bronneke J, Debatin JF, et al. Advancing digital health applications: priorities for innovation in real-world evidence generation. Lancet Digit Health. 2022;4(3):e200-e206. [DOI] [PubMed] [Google Scholar]
- 21. Selker HP, Eichler HG, Stockbridge NL, et al. Efficacy and effectiveness too trials: clinical trial designs to generate evidence on efficacy and on effectiveness in wide practice. Clin Pharmacol Ther. 2019;105(4):857-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerstein HC, McMurray J, Holman RR. Real-world studies no substitute for RCTs in establishing efficacy. Lancet. 2019;393(10168):210-211. [DOI] [PubMed] [Google Scholar]
- 23. Lauer W, Löbker W, Höfgen B. Digitale Gesundheitsanwendungen (DiGA): Bewertung der Erstattungsfähigkeit mittels DiGA-Fast-Track-Verfahrens im Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2021;64:1232-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Institute for Health and Care Excellence (NICE). Evidence Standards Framework (ESF) for digital health technologies. https://www.nice.org.uk/corporate/ecd7/resources/evidence-standards-framework-for-digital-health-technologies-pdf-1124017457605. Published December 10, 2018. Accessed June 3, 2022.
- 25. mHealthBELGIUM. Validation pyramid. https://mhealthbelgium.be/validation-pyramid. Date unknown. Accessed June 3, 2022.
- 26. mHealthBELGIUM. Belgian platform for mobile apps—all applications. https://mhealthbelgium.be/apps. Date unknown. Accessed June 3, 2022.
- 27. mHealthBELGIUM. First app in level M3 of validation pyramid. https://mhealthbelgium.be/news/persbericht-duidelijk-financieringskader-nodig-voor-doorbraak-medische-apps-in-belgie-4. Published April 18, 2022. Accessed June 3, 2022.
- 28. Lovell T. France to enable rapid market access for digital therapeutics. https://www.healthcareitnews.com/news/emea/france-enable-rapid-market-access-digital-therapeutics. Published October 20, 2021. Accessed June 3, 2022.
- 29. EIT Health. DiGAs—a model for Europe? Possible options for achieving an European system. https://eit-health.de/wp-content/uploads/2022/01/EITHEA2.pdf. Published September 16, 2021. Assessed June 3, 2022.
- 30. Kassenärztliche Bundesvereinigung (KBV). Telematikin frastruktur informationen zum anschluss der praxis, zur technischen austattung und zur finanzierung. https://www.kbv.de/media/sp/PraxisWissen_Telematikinfrastruktur.pdf. Published February 2019. Accessed June 3, 2022.
- 31. Eichler HG, Barker R, Bedlington N, et al. The evolution of adaptiveness: balancing speed and evidence. Nat Rev Drug Discov. 2018;17(12):845-846. [DOI] [PubMed] [Google Scholar]
- 32. Wenzl M, Chapman S. Performance-based managed entry agreements for new medicines in OECD countries and EU member states. https://www.oecd-ilibrary.org/social-issues-migration-health/performance-based-managed-entry-agreements-for-new-medicines-in-oecd-countries-and-eu-member-states_6e5e4c0f-en. Published 2019. Accessed August 24, 2022.
- 33. EIT Health. Implementing Value-Based Healthcare in Europe: Handbook for Pioneers (Director: Gregory Katz). Munich, Germany: EIT Health; 2020. [Google Scholar]
- 34. U S Food and Drug Administration (FDA). Digital health software precertification (Pre-Cert) program. https://www.fda.gov/medical-devices/digital-health-center-excellence/digital-health-software-precertification-pre-cert-program#program. Published June 5, 2021. Accessed June 3, 2022.
- 35. Jacobsen M, Dembek TA, Kobbe G, Gaidzik PW, Heinemann L. Noninvasive continuous monitoring of vital signs with wearables: fit for medical use. J Diabetes Sci Technol. 2021;15(1):34-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. EIT Health. EIT health innovation project. RealWorld4Clinic. https://eit-health.de/en/realworld4clinic/. Date unknown. Accessed June 3, 2022.
- 37. Nimri R, Battelino T, Laffel LM, et al. Insulin dose optimization using an automated artificial intelligence-based decision support system in youths with type 1 diabetes. Nat Med. 2020;26(9):1380-1384. [DOI] [PubMed] [Google Scholar]
- 38. Rivera SC, Liu X, Chan AW, et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. BMJ. 2020;370:m3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu X, Rivera SC, Moher D, et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. BMJ. 2020;370:m3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Phillip M, Bergenstal RM, Close KL, et al. The digital/virtual diabetes clinic: the future is now-recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23(2):146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]