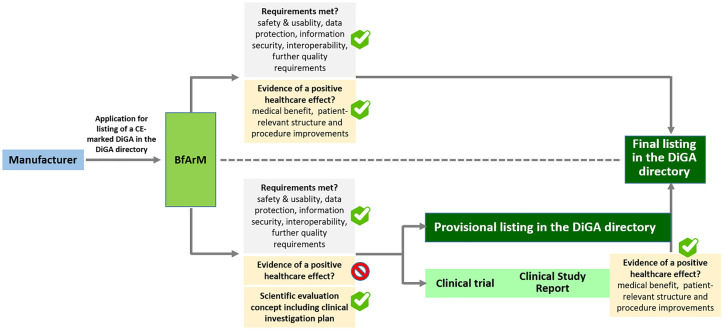
Figure 1.
The German fast-track procedure of a listing in the DiGA directory. Manufacturers apply for listing of their DiGA in the DiGA directory by submitting the package of required documents to the BfArM. In case all requirements including the demonstration of evidence of a positive healthcare effect are met, BfArM will grant a final listing of the DiGA in the DiGA directory within three months of receipt of the documents. In case all requirements are met, except from demonstration of evidence of a positive healthcare effect, BfArM can grant—also within a period of three months—a provisional listing of the DiGA in the DiGA directory. However, to receive a provisional listing, the manufacturer has to present a scientific evaluation concept including a clinical investigation plan prepared by an institution independent of the manufacturer. Manufacturers of provisionally listed devices need to provide evidence of a positive healthcare effect within a period of 12 months from the granting of the provisional listing. The timeframe can be extended once for another period of 12 months. Based on the trial outcomes documented in a clinical study report, BfArM evaluates the presented evidence of a positive healthcare effect within a period of three months. In the event of a positive evaluation result, BfArM approves the final listing of the DiGA in the DiGA directory. Through both the final and the provisional listing in the DiGA directory, the DiGA is covered by statutory health insurance nationwide and can be prescribed by any doctor or psychotherapist. Following the final listing in the DiGA directory, the manufacturer negotiates the amount of remuneration with the National Association of Statutory Health Insurance Funds (GKV-SV), which then replaces the previously applicable price set by the manufacturer. Abbreviations: DiGA, Digital health applications; BfArM, Federal Institute for Drugs and Medical Devices.