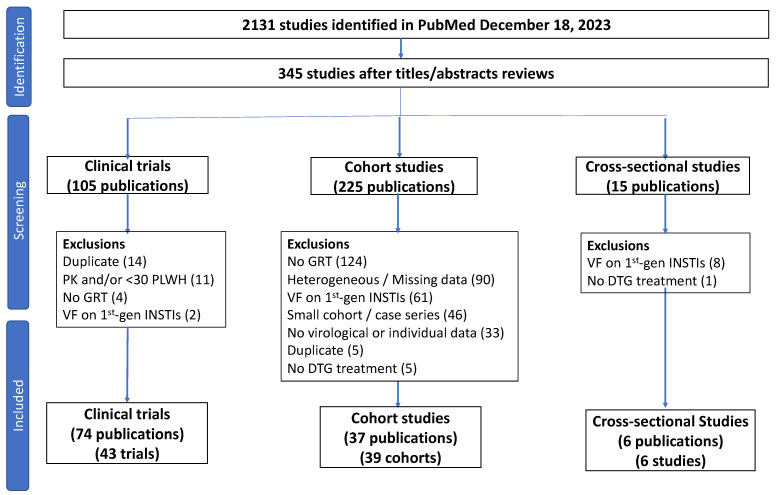
Figure 1.
Flow chart summarizing the review process. Of 2131 publications identified in the PubMed search, 345 were read in their entirety following an initial review of titles and abstracts. Exclusions for clinical trials and cross-sectional studies were usually based on a single exclusion criterion. Exclusions for cohort studies were usually based on more than one exclusion criterion. Two publications contained descriptions of two cohorts. Abbreviations: PK—pharmacokinetic study; PLWH—people living with HIV; GRT—genotypic resistance testing; VF—virological failure; 1st-gen INSTI—previous VF on a 1st-generation integrase strand transfer inhibitor (raltegravir or elvitegravir). Heterogeneous/Missing data—indicates that the study described different subsets of individuals with different ART histories, levels of virus suppression, and DTG-containing regimens but that the virological and/or GRT outcomes were not provided for the different subsets.