Figure 1.
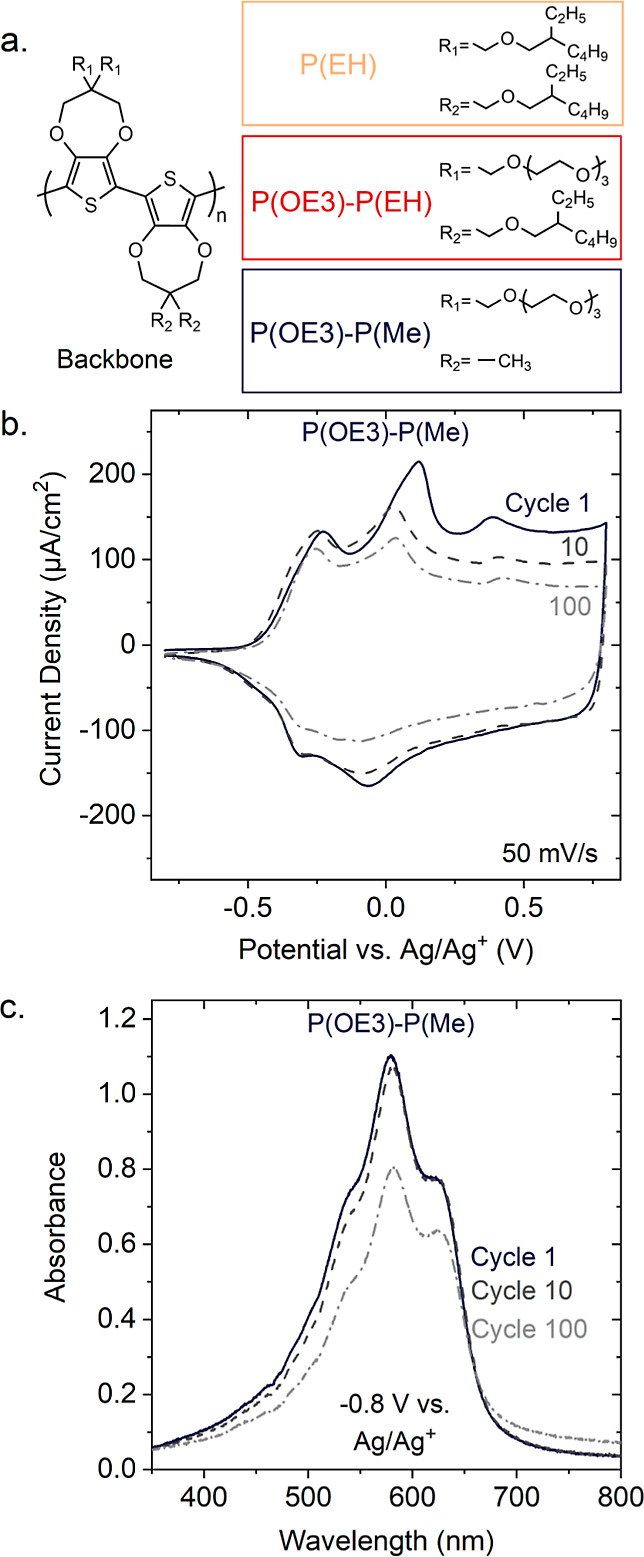
(a) Chemical structures of the poly(3,4-propylenedioxythiophene), P(ProDOT), derivatives investigated in this work. Left: P(ProDOT) backbone structure. Right: selected side-chain motifs. (b,c). Examples of durability issues in redox active polymers. (b) Cyclic voltammograms of P(OE3)-P(Me) during electrochemical cycling in 0.5 M tetrabutylammonium hexafluorophosphate (TBA+PF6–) dissolved in degassed propylene carbonate (PC), showing a decrease in the redox current upon repeated cycling. (c) Spectroelectrochemistry results for P(OE3)-P(Me) in the same electrolyte (0.5 M TBA+PF6–/PC) reveal a decrease in neutral-state linear absorbance upon repeated cycling, often referred to as “break-in”. Absorbance spectrum/cyclic voltammogram after one doping/dedoping cycle (solid lines); after 10 cycles (dark gray dashed lines); and after 100 cycles (light gray dash-dot lines).
