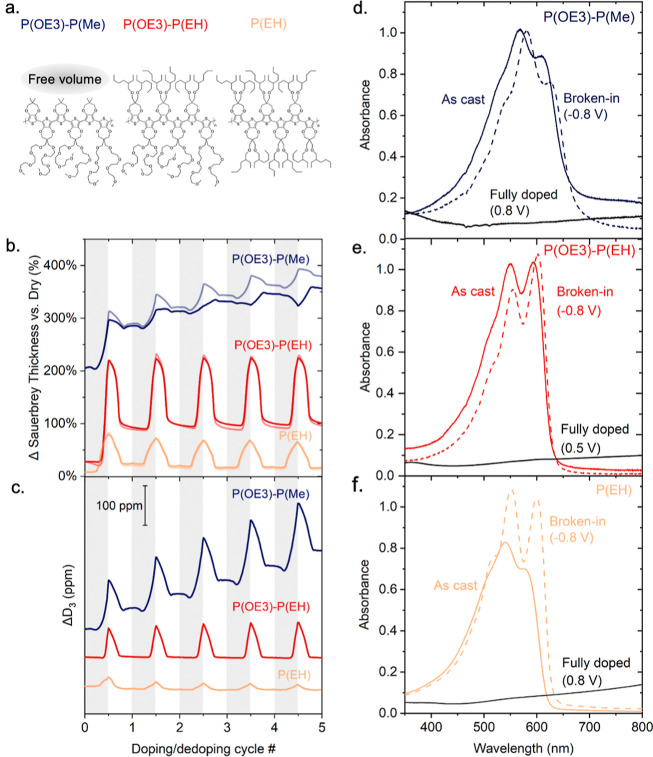
Figure 3.
Multiple electrochemical cycles show that the side-chain free volume in P(ProDOT)s affects ion sorption/desorption and optical “break-in”. (a) Illustration of the difference in side-chain free volume that is provided by the three P(ProDOT)s studied here, i.e., of the void space provided locally (on the length scale of a monomer unit) depending on the side-chain motifs selected, rather than the free volume provided over larger scales, e.g., due to limited molecular packing. (b) Estimated change in film thickness recorded for P(OE3)-P(Me), P(OE3)-P(EH), and P(EH) over multiple cycles. Lighter traces show changes estimated from the fundamental frequency shift; darker traces show those from the third overtone frequency shift. (c) Third overtone dissipation shifts, measured relative to the passively swollen state, reveal changes in softness and thickness due to active swelling (ΔD3 data are offset and stacked for clarity). Note: the gray vs white shades in panels (e and f) differentiate the doping (gray) from the dedoping scans (white). (d–f) UV–vis absorption spectra of, respectively, P(OE3)-P(Me), P(OE3)-P(EH), and P(EH): pristine (colored, solid lines), electrochemically oxidized, i.e., fully doped (black, solid lines), and electrochemically reduced (colored, dashed lines); potentials are given with respect to an Ag/Ag+ reference electrode (calibrated vs Fc/Fc+, E1/2 = +90 mV). The less pronounced 0–0/0–1 vibronic peak ratios in pristine P(EH) and P(OE3)-P(Me) are indicative of more H- and/or HJ-like photophysical coupling compared to that of the electrochemically doped state [note: a detailed discussion of the absorption line shapes of P(EH) and P(OE3)-P(Me) can be found, respectively, in ref (11) and refs (7 and 21). Moreover, the switching potential for P(OE3)-P(EH) was chosen at 0.5 V vs Ag/Ag+ as we observed some irreversibility in the redox switching beyond 0.65 V. Specifically, the neutral spectrum could not be fully recovered. There was no sign of degradation but rather some residual absorbance above 700 nm, which points to incomplete electrochemical reduction of the doped form and charge trapping. Film thicknesses were chosen to yield a broken-in film with a peak neutral state absorbance of ≈1.0].