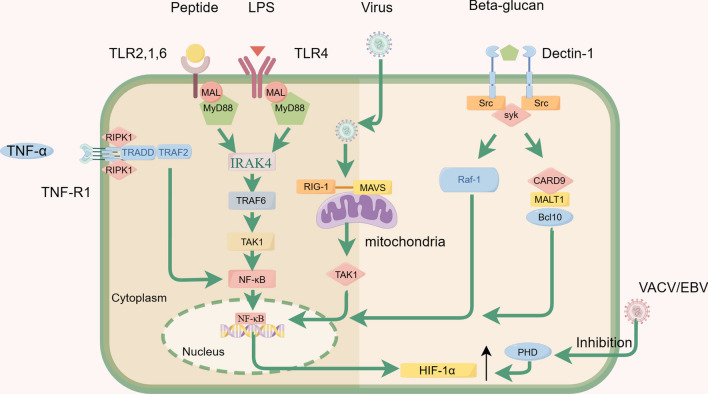
Fig. 2.
The infection of endothelial cells by pathogenic microorganisms triggers the activation of the NF-κB pathway, resulting in increased levels of HIF-1α. The figure mainly illustrates the pathways through which bacteria, viruses, fungi, and cytokines activate the NF-κB pathway: (1) The TLR activation by bacterial infection recruits the adaptor protein MyD88 (myeloid differentiation primary response 88) to propagate downstream signals. MyD88 subsequently activates a series of kinases, leading to the activation and nuclear translocation of nuclear factor-κB (NF-κB), a transcription factor that regulates the expression of several pro-inflammatory genes. (2) RIG-I plays a crucial role in initiating the innate antiviral immune response by serving as a key pattern recognition receptor for host recognition of viruses. RIG-I recognizes the RNA component of viruses and transmits signals by interacting with the downstream signaling molecule MAVS through its own CARD. This process activates the cellular transcription factors IRF-3 and NF-κB, allowing them to enter the nucleus. (3) Recognition of β-1–3-glucan in the fungal cell wall by dectin-1 enables the sensing of fungal pathogens and initiates host immune responses. Dectin-1 triggers the downstream signaling pathways of Syk and Raf1, which subsequently modulate the activation of both classical and non-classical NF-κB signaling pathways. (4) Upon activation by TNF-α, the TNFR1 triggers the formation of a signaling complex that induces a cellular response. In the assembly of Complex I, the activated TNFR1 binds to TRADD (TNFRSF1A-associated via death domain), followed by the interaction with a variety of components, such as receptor-interacting protein kinase 1 (RIPK1). This signaling pathway activates NF-κB and MAPK