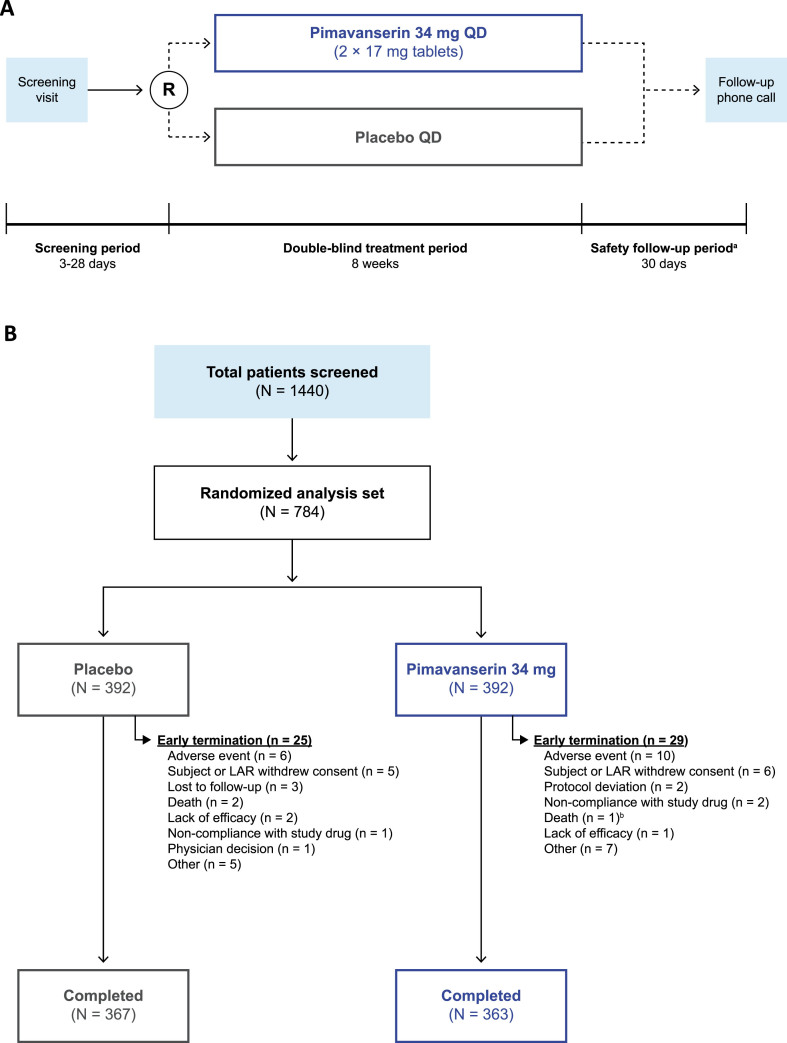
Fig. 1.
The (A) study design and (B) patient disposition of the trial. aSubjects who enrolled in the open-label extension study did not complete the safety follow-up period. bIn the pimavanserin group, one patient was discontinued from the study due to an adverse event and died 4 days after the early termination visit and 4 days after stopping study drug. LAR, legally acceptable representative; R, randomization; QD, once daily.