Figure S3.
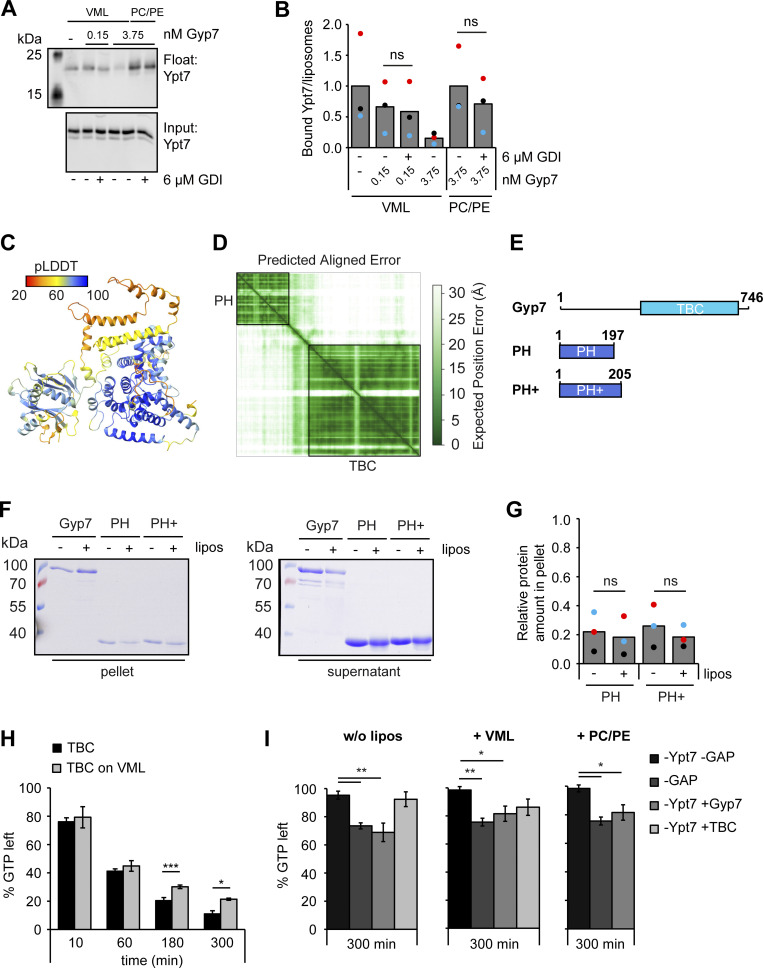
The N-terminal PH domain of Gyp7 does not bind membranes. (A) 250 μM PC/PE liposomes or liposomes of VML composition were preloaded with 0.6 μM Ypt7-GDI complex in the presence of 3.75 mM EDTA and 125 μM GTP. Nucleotide binding was stabilized by addition of 7.5 mM MgCl2. Reactions were incubated with different amounts of Gyp7 and, where indicated, with 6 μM GDI for 10 min. Liposomes were floated in a sucrose gradient. Control reaction contained respective liposomes of VML composition and no Gyp7. 40% of the float was analyzed together with 3% input by western blotting using an anti-Ypt7 antibody. (B) Quantification of bound Ypt7 to liposomes in A. Band intensity of Ypt7 signal in float was measured in Fiji and compared with input. Reactions containing Gyp7 were normalized to the average value of the control reaction. Bar graphs represent the averages from three independent experiments and dots represent the mean of each experiment. P value ns, using ANOVA one-way test. (C) AlphaFold2 structure prediction of Gyp7 color-coded according to the pLDDT values. (D) Plot of the Predicted Aligned Error of C with the PH and TBC domain of Gyp7 labeled. (E) Comparison of full-length Gyp7 and its PH domain. Two PH domain constructs (PH = aa 1–197; PH+ = aa 1–205) contain the N-terminal region of Gyp7 (aa 1–746). (F) Membrane association of the PH domain compared to full-length Gyp7. 715 nM protein was incubated with 715 μM liposomes of VML composition for 10 min. Membranes were separated from supernatant by centrifugation at 100,000 g and both fractions were analyzed by SDS-PAGE and Coomassie staining. Control reactions contained no liposomes. (G) Quantification of the relative protein amount in the pellet in F. Band intensity of protein signal in the pellet was measured in Fiji and compared with the protein signal in the supernatant. Bar graphs represent the averages from three independent experiments and dots represent the mean of each experiment. P value ns, using ANOVA one-way test. (H) Comparison of the Gyp7 TBC domain activity toward soluble Ypt7-GTP in solution and on membranes. 5 μM Ypt7 was incubated with 5 μM GAP and 50 μM GTP in the presence of 1 mM DTT, 20 mM EDTA, and 5 mM MgCl2. Where indicated, reactions contained 1 mM liposomes with VML composition. Control reactions contained no Ypt7, no GAP, or neither Ypt7 nor GAP (see Fig. S3 I). Reactions were stopped after 0, 10, 60, 180, and 300 min by snap-freezing and boiling at 95°C. Samples were applied to a HPLC system and the absorbance of GDP and GTP was monitored at 254 nm. Peaks were analyzed with OpenChrom and for each time point the percentage of GDP and GTP in the samples was determined. The percentage of GTP left at each time point was normalized to the respective percentage of GTP at t = 0 min. Normalized % GTP left plotted against the time in min. Bar graphs represent the averages and error bars the SD from three independent experiments. P value *<0.05, ***<0.001, using ANOVA one-way test. (I) No major GTP hydrolysis in control reactions of Fig. 5 J and H after 300 min. Control reactions contained no Ypt7, no GAP, or neither Ypt7 nor GAP. For each time point the percentage of GDP and GTP in the reactions was determined. The percentage of GTP left of each sample at t = 300 min was normalized to the respective percentage of GTP at t = 0 min. Normalized % GTP left plotted against the time in min. Bar graphs represent the averages and error bars the SD from three independent experiments. P value *<0.05, **<0.01, using ANOVA one-way test. Source data are available for this figure: SourceData FS3.