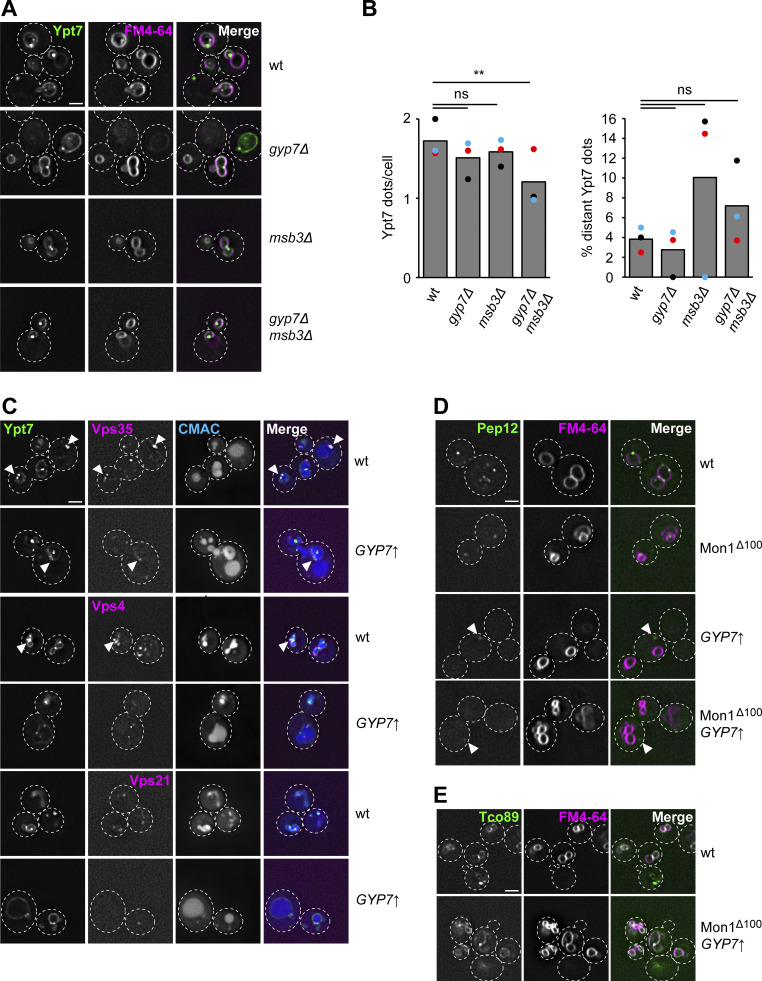
Figure S4.
Confined Ypt7 dots correspond to signaling endosomes. (A) Localization of mNeon-Ypt7 in wild-type (wt) cells and cells with Gyp7 and/or Msb3 deleted. Vacuolar membranes were stained with FM4-64. Cells were imaged by fluorescence microscopy. Individual slices are shown. Dashed lines indicate yeast cell boundaries. Scale bar, 2 μm. (B) Quantification of the total number of Ypt7 dots per cell and the percentage of distant Ypt7 dots in A. The number of distant Ypt7 dots (not at the vacuole) was divided by the total number of Ypt7 dots per cell. Cells (n ≥ 150) from three independent experiments were quantified in Fiji. Bar graphs represent the averages from three experiments and dots represent the mean of each experiment. P value ns, **<0.01, using ANOVA one-way test. (C) Localization of mNeon-Ypt7 dots relative to endosomal marker proteins. Endosomal markers Vps35-mKate, Vps4-3xHA-mCherry, and mCherry-Vps21 were co-expressed in TEF1pr-GYP7 or wild-type cells encoding endogenous mNeon-Ypt7. Vacuoles were stained with CMAC. Cells were imaged by fluorescence microscopy. Individual slices are shown. Arrowheads depict representative colocalization. Dashed lines indicate yeast cell boundaries. Scale bar, 2 μm. (D) Localization of GFP-Pep12 in wild-type cells and cells expressing Gyp7 from the TEF1 promoter and/or Mon1Δ100-Ccz1. Vacuolar membranes were stained with FM4-64. Cells were imaged by fluorescence microscopy. Individual slices are shown. Arrowheads depict distant Pep12 dots. Dashed lines indicate yeast cell boundaries. Scale bar, 2 μm. (E) Localization of Tco89-mNeon in wild-type and TEF1pr-GYP7 cells. Vacuolar membranes were stained with FM4-64. Cells were imaged by fluorescence microscopy. Individual slices are shown. Dashed lines indicate yeast cell boundaries. Scale bar, 2 μm.