Fig. 3 |. Suggested workflow for inclusion of study participants into preclinical Alzheimer’s disease trials.
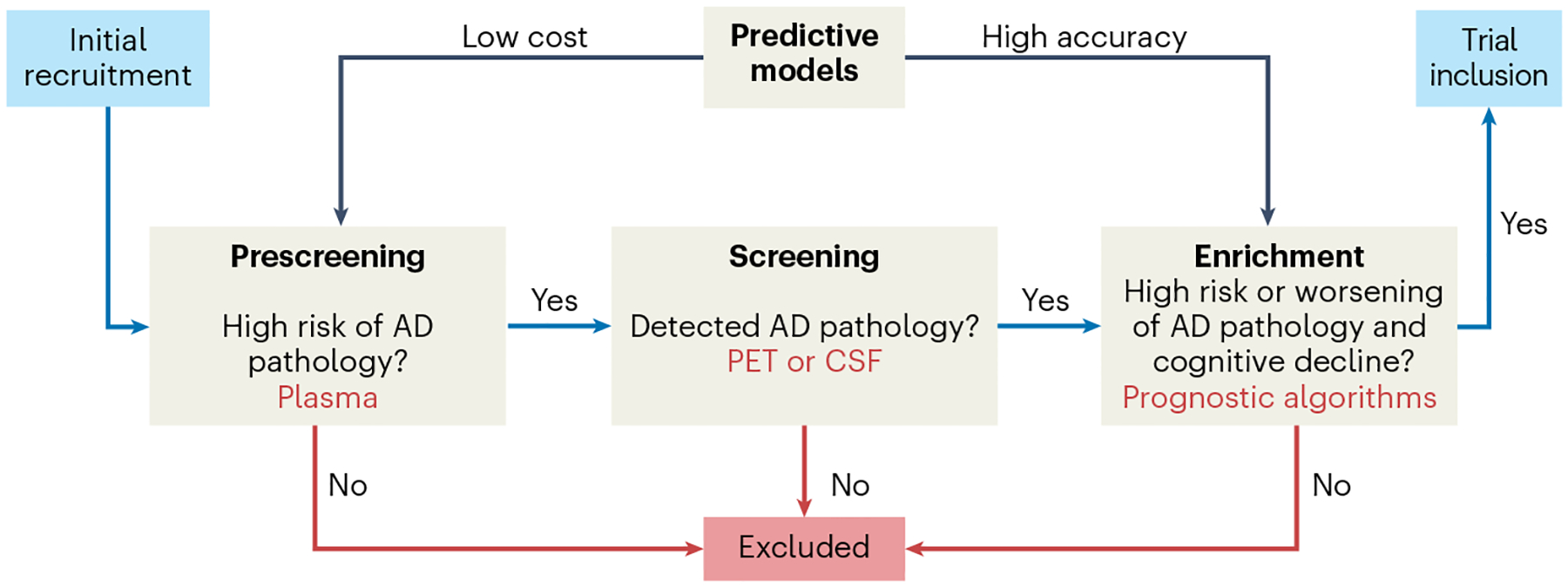
In the ‘prescreening’ step, a diagnostic algorithm based on blood-based biomarkers (BBMs) for Alzheimer’s disease (AD) identifies cognitively normal individuals as being at low risk or high risk of having pre-symptomatic (preclinical) AD. In the ‘screening’ step, individuals deemed high risk will undergo further tests, involving amyloid-β (Aβ) positron emission tomography (PET) or cerebrospinal fluid (CSF) AD biomarkers, to confirm or rule out the presence of AD pathology. The prescreening step with BBMs will result in substantial time and cost savings, as far fewer CSF or PET tests will be needed to identify a certain number of individuals with preclinical AD. In the ‘enrichment’ step, a prognostic algorithm can be used to identify individuals who are likely to subsequently exhibit more severe spread of tau pathology and cognitive decline, so that the population to be included in the trial is enriched for such individuals. This latter enrichment step enables preclinical AD trials with shorter durations and/or fewer study participants.
