Figure 1.
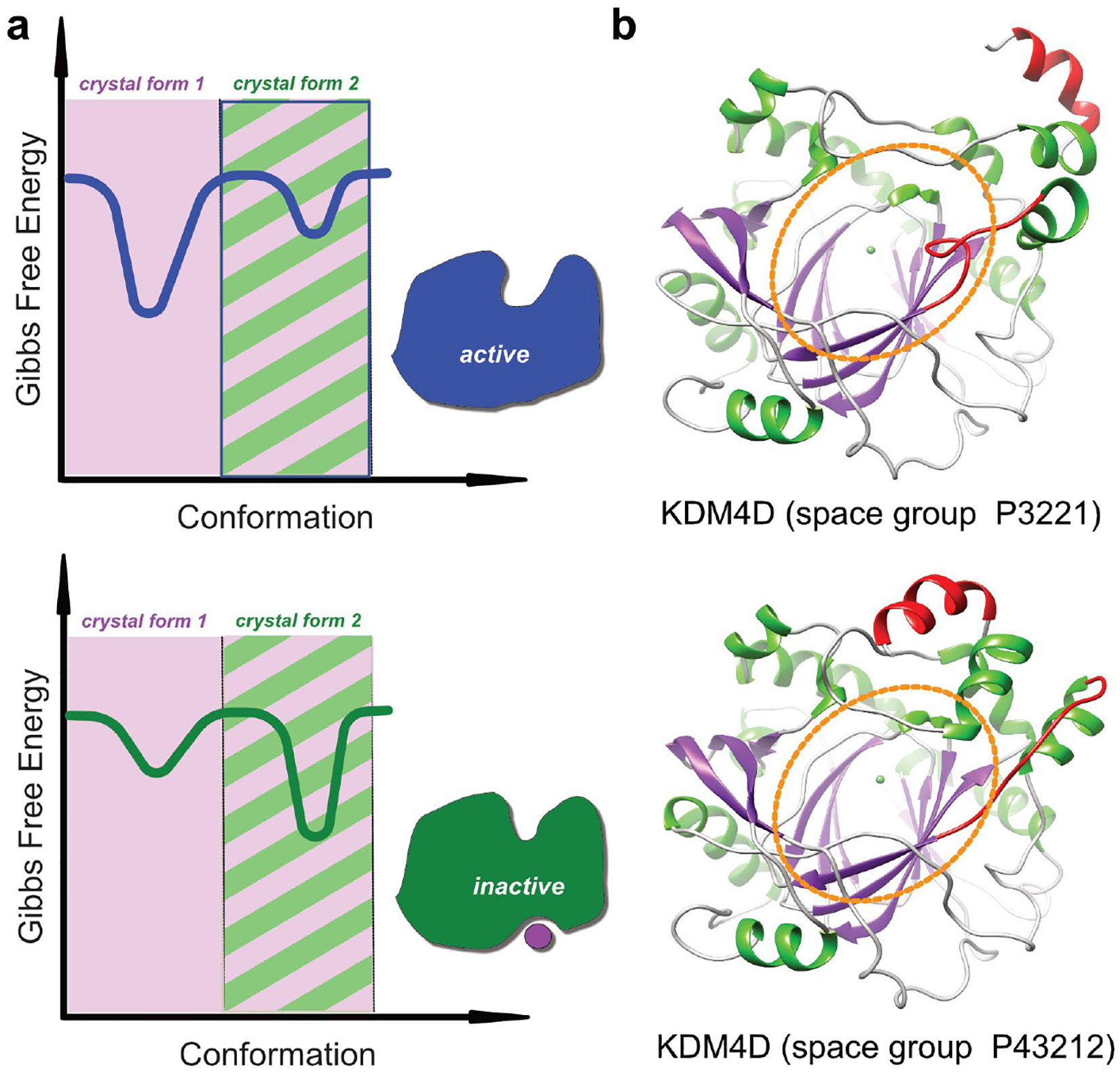
Accessible conformational landscape in protein crystals. (a) The plots show a schematic representation of the energy landscape. Binding of an allosteric effector molecule leads to a conformational change which shifts the energy landscape from the active (top) to the inactive form (bottom). The shaded regions indicate the accessible conformational space in two different crystal forms. In the example, crystal form 1 can accommodate conformational changes which accompany binding of an allosteric effector, whereas crystal from 2 can only accommodate one conformation. (b) Structure of human KDM4D in two different crystal lattices. KDM4D is a histone demethylase and the region encircled in orange indicates the active site pocket. The protein conformation crystallized in space group P3221 is identical with a structure of KDM4D in complex with H3K9Me3, whereas the conformation crystallized in space group P43212 has a distorted active site pocket and appears to represent an inactive conformation. Hence, any ligands that are able to stabilize this unusual conformation, whether by binding to the active site or to any other conformation-specific pocket, should in principle be able to serve as inhibitors. The conformation observed in space group P43212 is stabilized through a loop which is part of the active site (coloured in red), but which is involved in crystal contacts and which therefore does not allow conformational changes that would lead to a properly formed active site pocket.
