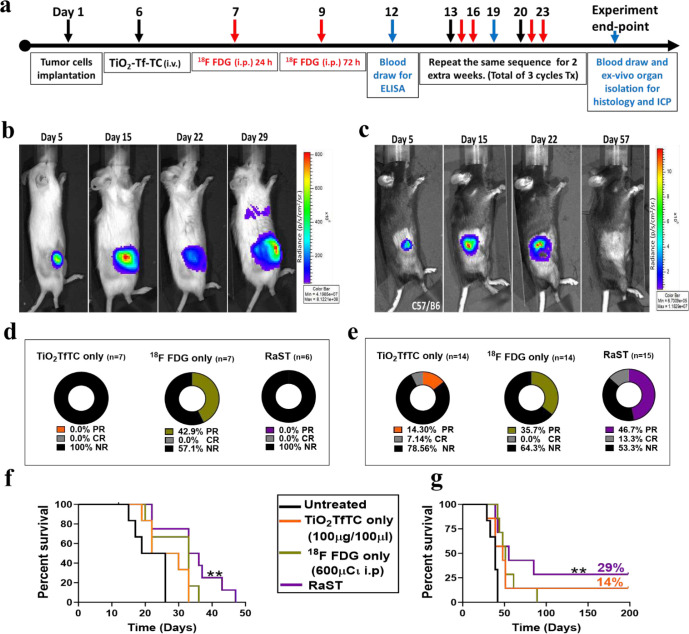
Fig. 4|. Assessment of cumulative treatment response using the immune-related response criteria.
(a) Schematic for the in vivo RaST study. (b) Representative BLI images of tumor progression on different days post-treatment using immunocompromised NSG mice inoculated in MFP with PyMT-BO1PyMT-BO1 cells. (c) Representative BLI images of tumor progression on different days post-treatment using immunocompetent C57BL/6 mice inoculated in MFP with PyMT-BO1 cells. (d-e) Cumulative RaST response assessment using the immune-related response criteria (irRC) categorized into no-response or progressive disease (NR), partial response (PR) and complete response (CR) in (d) NSG PyMT-BO1orthotopic MFP model: Untreated n=3, TiO2-TC-Tf n=5, 18FDG only n=6 and RaST n=6, and (e) C57BL/6 PyMT-BO1 orthotopic MFP model, Group A: Untreated n=6, TiO2-TC-Tf n=7, 18FDG only n=7 and RaST n=8 and Group B: Untreated n=6, TiO2-TC-Tf n=7, 18FDG only n=7 and RaST n=7. (f and g) The Kaplan-Meier survival plots of C57BL/6 mice inoculated with (f) 1.0 × 105 [Group A] and (g) 5.0 × 104 [Group B] PyMT-BO1 GFP Luc cells. A significant survival difference and improved overall survival were observed in the Group B cohorts.