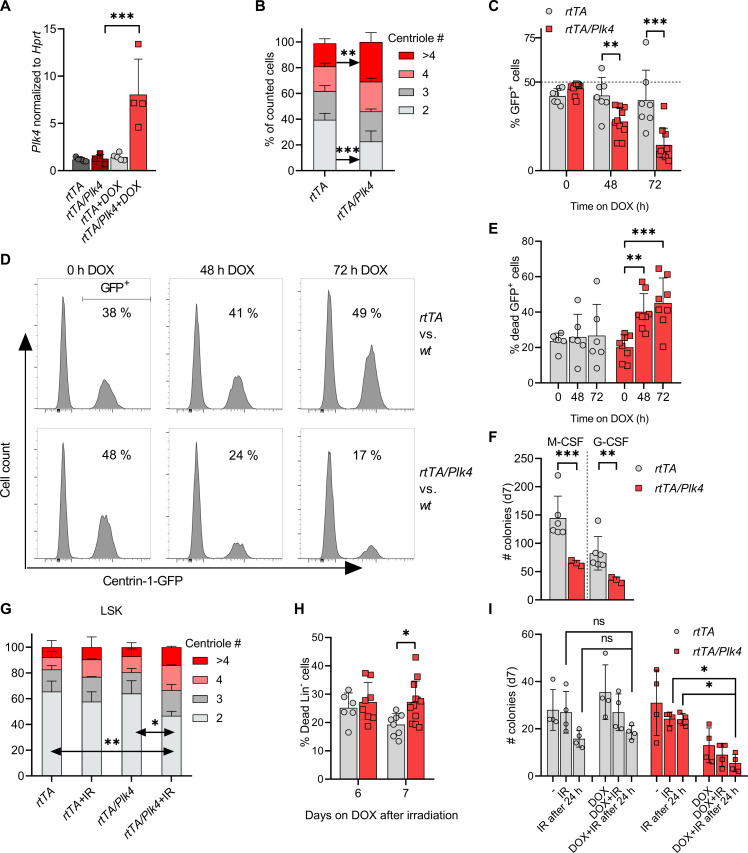
Fig. 5. PLK4 overexpression limits the clonogenic potential of HSPCs.
(A) Plk4 mRNA in MPP cells was assessed after 48 hours of culture ± doxycycline and normalized to Hprt (rtTA, n = 5; rtTA/Plk4, n = 4). (B) MPPs from rtTA (n = 5) and rtTA/Plk4 (n = 4) mice were cultured ± doxycycline for 48 hours to quantify centriole numbers. (C) Quantification of SMP competition assays, rtTA (two biological replicates, three technical replicates); rtTA/Plk4 (five biological replicates, two technical replicates). (D) Representative histograms of cell competition assays using SMPs from rtTA mice, mixed 1:1 with SMPs from rtTA or rtTA/Plk4 mice expressing also EGFP-Centrin1, quantified in (C). (E) Quantification of cell death observed in (C). rtTA (two biological replicates, three technical replicates), rtTA/Plk4 (four biological replicates, two technical replicates). (F) MethoCult assays using fetal livers from E14.5 embryos of rtTA (n = 6) and rtTA/Plk4 mice (n = 3), cultured in the presence of doxycycline. (G) Percentage of LSK cells presenting with 2, 3, 4, or >4 centrioles. Cells were isolated from rtTA (n = 2) or rtTA/Plk4 (n = 4) mice, fed with doxycycline for 5 days after 1.75-Gy IR. At least 160 cells were counted per condition. (H) rtTA and rtTA/Plk4 mice 4 weeks of age were exposed to 1.75 Gy of IR and kept on doxycycline until analysis. Lin-negative cells from bone marrow were analyzed by forward/sideward-scatter separation to estimate viability. (I) M-CSF–induced colony formation using total bone marrow from 6-week-old rtTA (n = 4) and rtTA/Plk4 mice (n = 4). Bone marrow was irradiated with 0.5 Gy before seeding or 24 hours after seeding. Doxycycline was applied immediately in both settings. Data are shown as means ± SD. qRT-PCR, fetal-liver MethoCult, and competition assay data were statistically tested by Tukey’s multiple comparisons test. BM-MethoCult and centriole quantification data were tested by Sidak’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.005.