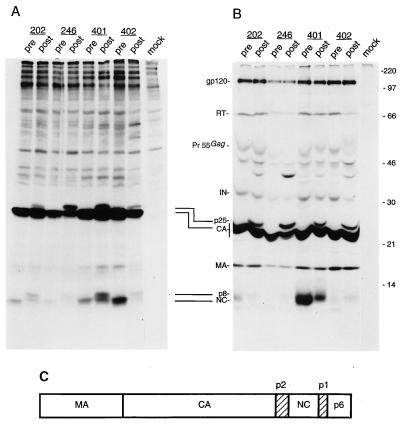
FIG. 4.
Analysis of the protein content of viral particles produced by recombinant viruses carrying pretherapy (pre) or postresistance (post) protease sequences from patients 202, 246, 401, and 402. (A) Metabolically 35S-labelled viral particles produced by transfected HeLa cells were partially purified by ultracentrifugation through a 20% sucrose cushion, lysed, and directly analyzed by SDS-PAGE. (B) Particle lysates produced and purified under the same conditions as for panel A were immunoprecipitated by serum from an HIV-1-infected patient and subsequently analyzed by SDS-PAGE. The amount of virions analyzed for each pair of pretherapy-postresistance viruses was normalized with an HIV-1 p24 antigen ELISA. Molecular mass markers (in kilodaltons) are shown on the right. On the left, the positions of the prominent HIV-encoded proteins found in virion preparations are indicated: gp120 (outer envelope glycoprotein), RT, IN (integrase), CA, MA, and NC. Pr55Gag indicates the complete, uncleaved Gag polyprotein precursor. Two incomplete Gag cleavage products, found solely in particles produced by recombinant genomes carrying postresistance protease sequences, are also indicated: p25, resulting from incomplete cleavage at the CA-p2 site; and p8, likely to result from incomplete cleavage at the NC-p1 site. (C) Schematic representation of the protein domain organization in the HIV Gag polyprotein precursor. The mature Gag proteins MA, CA, NC, and p6 are shown as open boxes; spacer peptide sequences p2 and p1 are shown as hatched boxes.