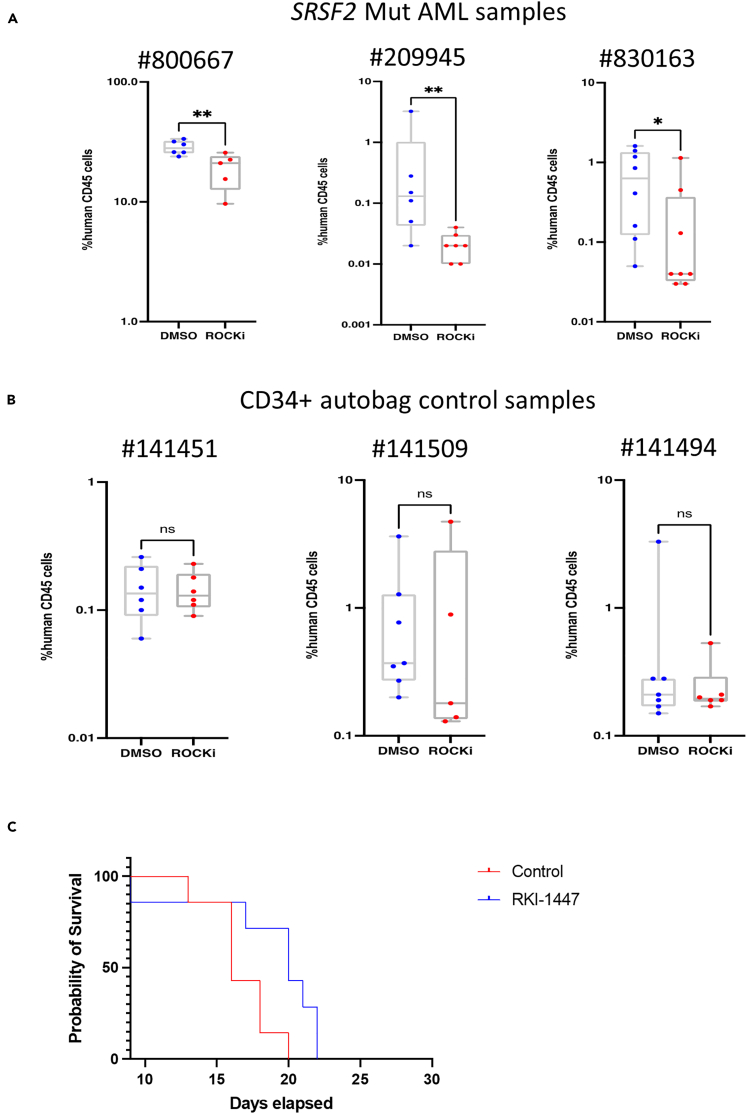
Figure 2.
in vivo efficacy and toxicity of RKI-1447
(A) CD3-depleted frozen PBMC from three SRSF2-mutated acute myeloid leukemia (AML) samples were injected into SGM3 (#800667) or NSG mice (#209945 and #830163) intrafemorally (i.f.); after 5 weeks transplantation mice were treated with RKI-1447 (50 mg/kg) or DMSO control for 21 days.
(B) NSG mice (n = 5–10/sample) were injected with 80,000 to 150,000 CD34+ cells from three mobilized peripheral blood samples (i.f.). On day 35 the animals were randomized to RKI-1447 or a carrier control. RKI-1447 was administered i.p. at a dose of 50 mg/kg daily for 21 days. On day 56 mice were sacrificed and analyzed for human CD45+ (hCD45) cells engraftment by flow cytometry. Mann-Whitney U test with FDR correction for multiple hypothesis testing, ∗p < 0.05; ∗∗p < 0.005; ∗∗∗p < 0.0005.
(C) 5 ∗ 10ˆ6 MOLM14 mutated cells were injected into 225 rad irradiated NSG mice. The mice were treated with RKI-1447 (50 mg/kg/day), starting from day 3 following cell transplantation. The mice were treated every day via intraperitoneal (i.p.) injection for 21 days. The Kaplan-Meier test p = 0.01.