Abstract
The binding of a cognate antigen to T cell receptor (TCR) complex triggers a series of intracellular events controlling T cell activation, proliferation, and differentiation. Upon TCR engagement, different negative regulatory feedback mechanisms are rapidly activated to counterbalance T cell activation, thus preventing excessive signal propagation and promoting the induction of immunological self-tolerance. Both positive and negative regulatory processes are tightly controlled to ensure the effective elimination of foreign antigens while limiting surrounding tissue damage and autoimmunity. In this context, signals deriving from co-stimulatory molecules (i.e., CD80, CD86), co-inhibitory receptors (PD-1, CTLA-4), the tyrosine phosphatase CD45 and cytokines such as IL-2 synergize with TCR-derived signals to guide T cell fate and differentiation. The balance of these mechanisms is also crucial for the generation of CD4+ Foxp3+ regulatory T cells, a cellular subset involved in the control of immunological self-tolerance. This review provides an overview of the most relevant pathways induced by TCR activation combined with those derived from co-stimulatory and co-inhibitory molecules implicated in the cell-intrinsic modulation of T cell activation. In addition to the latter, we dissected mechanisms responsible for T cell–mediated suppression of immune cell activation through regulatory T cell generation, homeostasis, and effector functions. We also discuss how imbalanced signaling derived from TCR and accessory molecules can contribute to autoimmune disease pathogenesis.
Keywords: TCR, regulatory T cells, immunological self-tolerance, CTLA-4, IL-2, CD45
One of the most important functions of the immune system is to mount a specific and efficient response against foreign antigens, but at the same time, since the uncontrolled immune cell activation could be dangerous to the host, this process must be finely tuned to limit and/or prevent excessive damage to surrounding tissues. In addition, immune cells need to maintain a state of immunological tolerance towards self-antigens to avoid the development of autoimmune diseases, suggesting the existence of a number of regulatory mechanisms controlling immune cell activation and response. The nature and outcome of T cell response are influenced by multiple pathways, including T cell receptor (TCR)-mediated signaling, which plays a central role in controlling T cell function and drives the immune response towards either effector or tolerogenic commitment. In this context, both the strength and duration of the TCR-mediated signals influence T cell activation and fate decision. TCR pathway engagement is triggered by the recognition of a specific peptide presented in the context of either class I or class II major histocompatibility complex (MHC) molecules expressed on the surface of antigen presenting cells (APCs) (first signal); together with this signal, co-stimulatory and co-inhibitory molecules expressed on the T cell surface transduce signals into the cells (second signal) that synergize with TCR signaling and positively or negatively modulate its pathway, leading to either activation, proliferation, and cytokine secretion or to anergy and apoptotic death, respectively (1). Interestingly, the expression of these co-inhibitory molecules is often induced upon T cell engagement in the presence of co-stimulation, suggesting the presence of a negative feedback loop controlling the immune system activation. The key role of these coinhibitory molecules in the control of immune cell activation is confirmed by the observation that their genetic ablation leads to the development of several autoimmune diseases, mainly associated with overactive T cell response (2). Activation of TCR signaling cascade is associated with a series of phosphorylation events triggered by the LCK–ZAP-70 axis. These events lead to the activation of downstream enzymes, including PLC-γ1, PI3K, and MAPK, with consequent propagation of the signal (3). The succession of phosphorylation and de-phosphorylation reactions is the most flexible and immediate tool for regulating the TCR-signaling cascade, although protein modifications, such as ubiquitylation and deubiquitylation, can also contribute to the net signaling output (4). In addition to the aforementioned mechanisms of TCR regulation, the affinity between the TCR and the MHC:peptide complex, their quantitative expression on the T cell surface, the timing and qualitative nature of their interaction can also control T cell function and determine their fate (5).
The regulatory negative mechanisms, that act rapidly and are generated in parallel with TCR-mediated activating signals, have been extensively characterized and can modulate T cell activation, ensuring the generation of appropriate responses to external stimuli. In this context, a recent paper by Chan et al. has shown that programmed cell death protein 1 (PD-1) plays a key role in TCR-induced signaling inhibition. Specifically, it has been reported that PD-1–mediated inhibition was able to exclusively target pathways activated by TCR engagement, independently of CD28 contribution, and it was highly sensitive to both the intrinsic quality of the ligand and the density of peptide:MHC complex on the surface of APCs (6).
It has now become clear that signals from the TCR and from co-stimulatory and co-inhibitory molecules are essential not only for limiting and directing T cell activation but also for controlling the development and suppressive function of CD4+ regulatory T (Treg) cells. This T cell–specialized subset expresses the transcription factor Foxp3 and is involved in the negative regulation of immune-mediated inflammation and in the maintenance of immune tolerance towards self-antigens (7). Several studies have revealed that also in the thymus, the strength and the affinity of interactions between TCR and MHC-bound self-peptides play a key role in driving T cell fate towards clonal deletion versus either maturation into conventional T (Tconv) cells or differentiation into thymic-derived Treg (tTreg) cells, also called “natural” Treg cells. Moreover, in the periphery, TCR-mediated signaling is also able to regulate the conversion of naive CD4+ T cells into the so-called “peripheral” Treg (pTreg) cells. However, other signals are also involved in the balance of these processes, as multiple T cell–associated factors can influence the overall strength of TCR signaling.
In this review, we will mainly focus on the most recent findings concerning the integration and coordination of distinct signals derived from the TCR, the co-stimulatory and co-inhibitory molecules (e.g., CD80, CD86, PD-1, B7-1, CTLA-4), the tyrosine phosphatase CD45 and cytokines (such as IL-2), with particular emphasis on their role in controlling the functional outcome of T cell responses and the homeostasis of Treg cells.
Role of TCR signaling in the control of immunological self-tolerance
TCR signaling is controlled by several mechanisms that differ in time of action and/or target molecules. The biological outcome of TCR binding is influenced by several parameters, including the avidity of the ligand, the duration of binding, and the presence and type of accessory molecules on APCs. In this context, several membrane-bound scaffold proteins and cytoplasmic adaptor molecules have been shown to turn off TCR signaling in T cells by recruiting protein and lipid kinases and/or phosphatases. Among the phosphatases, PTPN22 regulates TCR signaling by binding the c-SRC kinase and inhibiting LCK activity in effector and memory T cells (8). Consistent with this evidence, mutations altering the PTPN22–c-SRC kinase interaction are associated with increased phosphatase activity, a condition that increases the risk of autoimmune diseases (9). Similarly, in the absence of the cytoplasmic protein tyrosine phosphatase SHP1, there is increased positive and negative thymocyte selection and T-cell activation resulting in the development of autoimmunity (10). Another mechanism of regulation of TCR activation is related to the half-life of the MHC:peptide complex interaction with the TCR (11) and indeed, mutations in the peptide (reducing this interaction) are associated with a disproportionate reduction in TCR-mediated signaling (12). This mechanism prevents TCR activation induced by its interaction with weak ligands and conversely allows signal transmission only in response to receptor–ligand interactions above a certain strength and threshold (13).
It is important to emphasize that the biological outcome of T cell response is also related to the intensity of the signal deriving from TCR stimulation by the peptide:MHC complex, which can generate an oscillatory, sustained, or transient response. In particular, oscillatory responses, which are for example induced by weak ligands in double-positive (DP) thymocytes, promote cell survival (14), whereas strong stimuli induced by agonist peptide:MHC complexes can lead to DP thymocyte death (15). On the other hand, lower amplitude but longer lasting signals produced by weaker stimuli, such as those deriving from antagonist or weak agonist peptide:MHC complexes, can induce DP thymocyte differentiation (15). In addition, suboptimal stimuli can induce T cell anergy or Treg cell generation (16). In summary, gene expression profiles and defined biological outcomes are likely to be determined by the amplitude and timing of TCR-mediated signals.
Role of TCR signaling in thymic Treg cell generation
Several lines of research have supported the key role of TCR-mediated signaling in the control of T cell fate and their differentiation towards effector or regulatory compartment. Specifically, both TCR-ligand affinity and signal strength have been shown to regulate the generation and function of both tTreg and pTreg cells. At the central level, the affinity of TCR for self-peptide is fundamental in controlling positive selection of CD4+CD25+ thymocytes; indeed, TCRs with low peptide affinity preclude CD25+ development, while higher TCR affinity for self-peptide promotes the thymic selection of CD4+CD25+ Treg cells (17). The analysis of a panel of TCRs with a broad range of reactivity toward a given antigen revealed that a TCR with higher affinity was associated with an increased absolute number of tTreg cells (18). As concerns the role of TCR-dependent signal strength, tTreg cell precursors are induced by stronger TCR signals than Tconv cells (19); in addition, it has been reported that the generation of Foxp3+ tTreg cells can be sustained only by the binding of a self-peptide agonist to a strong reactive TCR. On the contrary, the use of a partial self-peptide agonist resulted in thymocyte deletion with failure in Treg cell generation, thus suggesting that TCR-mediated signal quality is able to guide the fate of autoreactive thymocytes toward their elimination or differentiation into Treg cells (20). TCR affinity is a critical factor also for tissue-specific Treg cell function during autoimmunity; indeed, it has been observed that high expression of Treg-associated markers, such as T-bet, GITR, CTLA-4, and ICOS, was strongly correlated with TCR signal strength (21). However, although Treg cells are characterized by a more autoreactive TCR as compared to Tconv cells, both high- and low-affinity Treg cells contributed to protection from autoimmune diabetes, albeit through different mechanisms. Specifically, it was observed that Treg cells with a high-affinity TCR were characterized by increased expression of IL-10, TIGIT, GITR, and CTLA-4, while those with low affinity showed increased transcripts for Areg and Ebi3. These data suggested that the degree of TCR affinity for self-antigen may influence the mechanisms by which autoimmunity is controlled (22).
Although the precise signaling pathways governing TCR-mediated Treg commitment still remain enigmatic, recent findings have elucidated some of the mechanisms by which different signaling molecules can modulate the strength of the TCR signal, thus controlling Treg cell generation. In this context, O'Hagan et al. have demonstrated that the actin cytoskeletal remodeling protein Pak2 played a key role in tTreg cell development by controlling the strength of the TCR signal; indeed, Pak2−/− thymocytes exhibited reduced TCR signaling, and mice with T cell–specific deletion of this molecule were characterized by reduced number of tTreg cells and developed spontaneous autoimmune colitis. Furthermore, Pak2 deficiency in thymocytes has been associated with impaired STAT5 activation following IL-2 stimulation, suggesting that, in addition to the generation of strong TCR signals, Pak2 also regulated tTreg cell development by controlling IL2-mediated signals (23).
A growing body of evidence has revealed that perturbation of TCR downstream pathways and dysregulation of their key enzymes are associated with pathological conditions, such as immunodeficiency and autoimmunity (Fig. 1). Specifically, loss of ZAP-70 results in severe immunodeficiency associated with impaired T cell maturation in the thymus and their reduced activation in the periphery (24); at the same time, single-nucleotide substitution of ZAP-70 is associated with autoimmunity. Indeed, mice expressing a phosphorylation-defective mutant of ZAP-70, with consequent attenuation of TCR signaling, spontaneously developed autoimmune arthritis and were characterized by an altered negative selection of self-reactive T cells, together with a reduced number of tTreg cells with an impaired suppressive function (25, 26). In line with these studies, mice with ZAP-70 mutations resulting in either reduction of its levels or its inability to recruit TCR downstream effector molecules were characterized by impaired tTreg cell generation and altered deletion of autoreactive T cells with no impact on thymic differentiation of CD4+ and CD8+ T cells (27, 28) (Fig. 1). Several ZAP-70 amino acid substitutions, leading to a stepwise reduction in TCR-mediated signaling, can lead to autoimmune or immunocompromised conditions, depending on the reached signaling threshold, which is responsible for the opposing effects of TCR-mediated pathways on thymic selection and immunological self-tolerance establishment. Specifically, a ZAP-70 variant that can only moderately reduce TCR signaling has been shown to exert no effect on thymic selection, while a more severe ZAP-70 defect abolished thymic positive selection, leading to immunodeficiency. Crossing these two mutations had a more consistent effect on the process of thymic negative selection and the generation of tTreg cell, whose impairment resulted in excessive autoantibody and hyper-Immunoglobulin E production (29). Alterations in ZAP-70 function that dampen TCR signaling can be at the basis of several autoimmune diseases by altering positive selection of those thymocytes that should be physiologically eliminated. However, attenuation of the TCR signal associated with ZAP-70 alterations has a greater impact on the Treg cell compartment than Tconv cells, since Foxp3 physiologically represses ZAP-70 expression, further inhibiting TCR signaling; as a result, reduction of TCR signaling intensity generates self-reactive Tconv cells and simultaneously impairs the development and function of Treg cells (30). Recent studies have shown that the activity of ZAP-70 as a scaffold protein, rather than its catalytic activity, is required for Treg cell function; specifically, TCR activation causes ZAP-70 phosphorylation, which results in the activation of a series of proteins that recognize its phosphorylation sites, enhancing Treg/Tconv interaction, thus promoting Treg cell suppressive activity (31) (Fig. 1).
Figure 1.
Schematic representation of the main alterations in TCR signaling pathway and accessory molecules associated with autoimmunity/immunodeficiency. The major signaling pathways induced by TCR engagement, combined with those derived from CD28 costimulatory molecule, CTLA-4 inhibitory receptor, the tyrosine phosphatase CD45 and IL-2R, involved in the control of T cell activation, and establishment of immunological self-tolerance. In the gray boxes are listed the alterations/pathological conditions associated with dysfunctions of the reported molecules. TCR, T cell receptor.
Another protein involved in TCR-dependent pathway and associated with both immunodeficiency and autoimmunity is represented by LCK, which is a Src family kinase pivotal for T cell activation and acquisition of effector functions. Recent findings have shown that complete deletion of LCK causes an immunodeficient phenotype, whereas partial attenuation of LCK function allows the maturation of Tconv cells but not regulatory T cells, leading to intestinal inflammation (32).
Together with LCK, also other TCR-associated molecules play a role in regulating tTreg cell generation: in particular, a knock-in mutation of LAT protein at Tyr136 leads to a lymphoproliferative syndrome with a severe deficit of Treg cells both in the thymus and in peripheral lymphoid organs (33). Similar results have been also observed in other experimental models, showing that LAT mutations (altering its interaction with PLC-γ1) inhibited Foxp3 expression together with Treg cell development and suppressive function, leading to severe autoimmune disease (33, 34). In line with this evidence, Fu et al. have shown that mice with a T cell–specific deletion of PLC-γ1 were characterized by impaired generation and function of Treg cells and developed autoimmune disease; on the contrary, enhancement of diacylglycerol-mediated signaling, a second messenger produced by activated PLC-γ, has been shown to increase Treg cell suppressive activity (35, 36) (Fig. 1).
Accordingly, signals that negatively regulate TCR-mediated pathways have opposite effects on Treg cell generation. Indeed, deletion of the tyrosine phosphatase Src homology region 2 domain–containing phosphatase-1 (SHP-1), a known negative regulator of TCR-mediated signaling, or exposure to increasing concentrations of stimulating peptide, resulted in an increased percentage of CD4+Foxp3+ cells in the thymus (37), and similar results were also found in mice bearing a deletion of CD5, another negative regulator of TCR signaling (38). Although the above data showed that attenuation of the TCR signaling strength correlates with decreased tTreg cell generation, it has recently been reported that mice with a mutant ζ chain (lacking functional ITAMs) displayed impaired negative selection of autoreactive thymocytes on the one hand and a significant increase in tTreg cell number on the other. At the molecular level, the described ζ chain mutation has been associated with reduced phosphorylation of ERK, Cbl, and protein kinase B or Akt, with little effects on calcium mobilization and activation of c-Jun N-terminal kinase, p38, and nuclear factor kappa-light-chain-enhancer of activated B cells, thus suggesting a selective and different role of ζ ITAMs on specific TCR-downstream pathways. In this context, the increased generation of Treg cells in these mutant mice could be related to specific attenuation of pathways able to inhibit Foxp3 expression, such as Akt (39). These data suggest that both inhibitory and activating molecules co-exist in TCR signaling pathway and participate in the control of tTreg cell generation with a different activation threshold. Specifically, the induction of tTreg cell is sustained by a strong but transient TCR activation mediated by high affinity ligands, according to the "hit and run" model (40, 41), which suggests that strong, but short-lasting TCR stimulation induces Foxp3-activating molecules and inhibits negative regulators of Foxp3 (such as Akt); in contrast, strong but long-lasting TCR-activating signals promote T cell clonal deletion (Fig. 2). Accordingly, thymic CD4+ T cells whose TCR is stimulated by poorly expressed and high affinity self-antigens, after transient activation, undergo a resting period in which they upregulate Foxp3 expression (40, 41).
Figure 2.
TCR signaling and tTreg cell differentiation. Thymic Treg cell differentiation from CD4 single-positive (SP) cells is sustained by a strong but transient TCR activation mediated by high affinity ligands, according to the “hit and run” model; in contrast, strong but long-lasting TCR-activating signals promote T cell clonal deletion. TCR, T cell receptor; Treg, regulatory T cell; tTreg, thymic-derived Treg cell.
These data are consistent with the observations of Jun et al. reporting that TCR activation can induce differentiation of CD4+ T cells into proinflammatory or regulatory T cells, depending on the molecular mechanism by which p38 kinase is activated after antigenic TCR stimulation. Specifically, TCR-mediated activation of p38 has been shown to occur through phosphorylation mediated by “classical” kinases or through ZAP-70–dependent “alternative” mechanisms; perturbation of the classical or alternative pathways had opposite effects on CD4+ T cell differentiation. Indeed, attenuation of the classical pathway led to altered IL-2 production, reduced Treg cell differentiation, and exacerbation of experimental autoimmune encephalomyelitis whereas, the alternative pathway was preferentially required for T helper (Th) 1 and Th17 pro-inflammatory functions (42) (Fig. 3). Another mechanism by which high-affinity TCR signals can promote Treg cell development involves members of the tumor necrosis factor receptor superfamily. Indeed, developing thymocytes, characterized by TCRs with high affinity for self-ligands, exhibited increased expression of GITR, OX40, and tumor necrosis factor receptor-2, which enhanced their ability to compete for limited amounts of IL-2, thus providing a selective advantage for their differentiation into Treg cells (43).
Figure 3.
The coexistence of the classical and alternative p38 activation pathways is necessary to maintain a balance between pro-inflammatory and anti-inflammatory responses. TCR stimulation activates two distinct p38 signaling pathways: the ‘classical’ pathway involving ZAP-70 kinase, LAT signalosome assembly, SOS recruitment, and activation of MAPK cascades, and the ‘alternative’ pathway, in which p38 is directly activated by ZAP-70. Indeed, TCR-induced ZAP-70 activation mediates p38 phosphorylation at Tyr323, inducing its auto-activation. Activation of p38 through the ZAP-70–dependent alternative pathway lowers the activation threshold for the classical pathway, leading to full p38 activation. Inhibition of the classical p38 pathway by SOS deletion leads to a reduction in Treg cell differentiation, whereas the alternative pathway is required to maintain pro-inflammatory T helper functions, thus suggesting that both pathways work in concert to balance pro- and anti-inflammatory T cell responses. TCR, T cell receptor; Treg, regulatory T cell.
Role of TCR signaling in peripheral Treg cell induction
The intrathymic generation of Treg cells mediated by the above-described processes does not appear to be the only way in which these cells can be obtained, as suppressor T cells can also derive from the conversion of naive CD4+CD25− T cells in the periphery. TCR-mediated signaling pathways also play a central role in regulating the induction and expansion of pTreg cell and are required to sustain the transcription of genes specifically expressed in activated Treg cells, such as interferon regulatory factor (IRF4) (44, 45). In addition, it has been shown that continuous TCR signals in the periphery are required to sustain Treg cell activation and suppressive function (46), but also weak TCR stimulation can promote their generation. Specifically, a low dose of a high-affinity TCR ligand represents the optimal quantitative and qualitative signal to induce a persistent population of Treg cells in vivo; moreover, also a chronic low-dose antigen exposure is capable of generating pTreg cells from naive T cells that are phenotypically and functionally similar to those generated in the thymus (47, 48).
Miskov-Zivanov et al. reported that there is an optimal “time window” of TCR stimulation for pTreg cell differentiation. The authors developed a logical network model of TCR signaling aimed at investigating the role of antigen dose and time of stimulation in determining T cell fate. Specifically, they found that restricting the time of TCR activation (achieved by antigen removal) resulted in three stable T cell fates (Th, Treg, and inactive) with relative proportions depending on the duration of stimulation (49). In the periphery, Foxp3 is induced more efficiently when T cells are stimulated under “sub-immunogenic” conditions (by low antigen doses with suboptimal dendritic cell (DC) activation), whereas stimulation with high doses of antigen results in increased T cell proliferation but reduced conversion into Foxp3+ cells, suggesting the presence of an inverse correlation between the number of cell divisions and the efficiency of Foxp3 induction (50). At the molecular level, this inverse correlation could be related to the cell cycle–dependent recruitment of DNA methyltransferase 1 and the subsequent CpG DNA methylation, resulting in the proliferation-dependent loss of Foxp3 expression (51). In support of this evidence, attenuation of TCR signaling strength by inhibition of ITK (a kinase involved in the TCR-induced pathway) has been shown to impair Th17 differentiation on one side, sustaining Treg cell differentiation on the other. ITK-deficient CD4+ T cells exhibited reduced TCR-induced phosphorylation of mTOR targets, such as Akt and ribosomal protein S6, resulting in reduced expression of hypoxia-inducible factor-1α and alterations in intracellular glycolytic metabolism (52) (Fig. 1). These data were consistent with previous experimental observations showing that inhibition of mTOR or Akt in CD4+ T cells promoted Foxp3 expression and Treg cell generation via multiple mechanisms (53, 54). The process by which TCR-mediated signaling strength promotes the induction of either Th or Treg cells involves the control of Akt activity. Specifically, depending on the strength of the TCR signal, Akt can interact with different substrates determining alternative cell fates (55). At the chromatin level, continuous TCR signaling and constitutive activation of the PI3K–Akt–mTOR pathway results in loss of the “permissive chromatin mark” for potential Foxp3 expression; in contrast, premature suspension of TCR signaling and inhibition of PI3K or mTOR pathways result in Foxp3 induction associated with permissive posttranslational histone modifications, such as di- and tri-methylation of histone H3 lysine 4 (H3K4me2 and -3) near the Foxp3 transcription start site and within the 5′ UTR (56). Consistent with this finding, Turner et al. have shown that functionally suppressive Foxp3+ Treg cells can be both induced and expanded in vitro by DCs presenting low doses of antigen and thus inducing weak TCR signaling. In contrast, exposure to high doses of antigen resulted in expansion of Foxp3 cells, characterized by strong activation of the Akt–mTOR pathway (57). Advances in the understanding of TCR-mediated signal transduction pathways and how these signals are differently regulated in Treg cells versus other T cell subsets could be exploited to selectively target Treg cells, with the aim of developing novel therapeutic regimen for cancer and autoimmune diseases.
CD28–CTLA-4 pathway involvement in the regulation of T cell activation
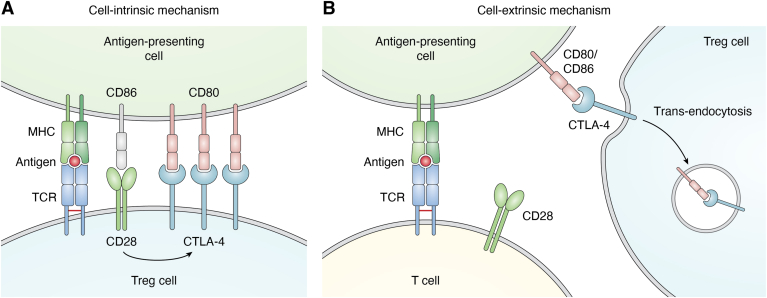
As previously mentioned, regulation of TCR-mediated signals also involves co-stimulatory/co-inhibitory molecules, such as CD28 and CTLA-4, which balance protective immunity, regulating immunological self-tolerance, and tissue damage. CD28 is a 44 kDa type I transmembrane protein expressed on the surface of the majority of naïve CD4+ and CD8+ T cells and represents the major costimulatory molecule required, together with TCR engagement, for the generation of effector T cell responses and immunological memory. More specifically, CD28 stimulation, achieved through its interaction with CD80 and CD86 ligands expressed by APCs, triggers and coordinates a series of metabolic pathways sustaining the energetic demand for rapid cell divisions (58). Conversely, TCR engagement in the absence of co-stimulation results in functional inactivation of T cells upon antigen encounter, leading to a vital but unresponsive state known as T cell anergy, a mechanism that contributes to the maintenance of peripheral self-tolerance (59, 60). The level of CD28 co-stimulation plays a key role in T cell decision process following antigen encounter, as high levels of co-stimulation lead to T cell activation against potential pathogens, whereas a weak CD28 co-stimulation induces T cell anergy and peripheral tolerance to self-antigens. Regulation of CD28 pathway activation is strictly dependent on the expression of both CD28 and CTLA-4 on T cells and CD80, CD86, and MHC on APCs (61). CTLA-4 (CD152) is a type I transmembrane glycoprotein homologous to CD28, with whom it shares a conserved hexameric motif MYPPPY, part of the ligand-binding site (62). While CD28 is constitutively expressed by T cells, CTLA-4 expression is induced only upon activation and both molecules are able to interact with CD80 and CD86 ligands expressed on APCs, albeit with different affinity. Unlike CD28, the binding of CTLA-4 to its ligand generates an inhibitory signal that prevents T cell activation (63). The sharing of the same ligands suggests that the functions of CD28 and CTLA-4, although opposite, are closely interconnected and finely tuned to control T cell activation. The signals mediated by these molecules are controlled by the relative expression levels of CD28 and CTLA-4 on one hand and by the binding affinity for their shared ligands on the other, with CTLA-4 having a significant higher affinity for both CD80 and CD86 than CD28 (64). The interplay between CD28 co-stimulation and CTLA-4 immunosuppressive function plays a pivotal role in the control of autoimmune responses and the maintenance of immunological self-tolerance. CD28 and CTLA-4 cooperation is also required for proper thymic selection of natural Treg cells and for deletion of autoreactive T cells. Thymocyte negative selection occurs upon high-avidity engagement of the TCR by self MHC-peptide complexes, in the presence of additional signals such as CD28 co-stimulation (65, 66, 67, 68), although some papers have failed to highlight an indispensable role of CD28 in the negative selection process (69, 70). Furthermore, CD28-mediated pathway not only modulates the strength of TCR signaling but also coordinates distinct signals delivered to the TCR to drive Treg cell precursor maturation and prevent their clonal deletion (71, 72). CTLA-4, in turn, shapes the TCR repertoire of autoreactive T cells during thymic selection and ensures that T cells with high TCR avidity can be selected to become Treg cells (73). More specifically, CTLA-4, expressed by the corticomedullary region of the thymus, has been shown to attenuate the strength of TCR-mediated signals, counteracting the effects of CD28 and preventing the elimination of those clones that should be eliminated due to their high affinity for self-antigens. This process plays a crucial role in modulating the level of TCR avidity required for thymic selection and, consequently, for Tconv and Treg cell development (73). Indeed, although CD28 costimulatory pathway is required for optimal T cell activation and therefore has been implicated in the induction and progression of autoimmune diseases, it is also required for the generation and the homeostasis of Treg cells. Several studies performed in mice showed that CD28 inhibition led to a reduction in Treg cell number with important implications for allograft rejection (74). Moreover, CD28-deficient Treg cells expressed low levels of CTLA-4, PD-1, and C-C chemokine receptor type 6, displaying impaired suppressive function (75, 76). In parallel, Treg cells in CTLA-4–deficient mice were highly proliferative, consistently with a strong CD28 co-stimulation (not counterbalanced by the inhibitory activity of CTLA-4) although functionally impaired, thus leading to an uncontrolled T cell activation (77, 78, 79) (Fig. 1). Accordingly, CTLA-4–deficient mice developed a lethal autoimmune lymphoproliferative disorder, and different experimental approaches aimed at blocking CD28 pathway ameliorated their pathological phenotype (58, 80) (Fig. 1). All these data suggest that the establishment of central tolerance as well as the maintenance of peripheral tolerance are strictly dependent on both CD28 and CTLA-4 pathways and, even more, on their mutual counterbalancing interplay. Several molecular models have been proposed to describe the antagonistic role of CTLA-4 towards CD28 signaling. Specifically, CTLA-4 is able to hamper CD28 signaling also via a “cell-extrinsic” mechanism (Fig. 4) in which CTLA-4 can capture CD80 and CD86 ligands from the cell surface via trans-endocytosis with their consequent degradation, resulting in the inhibition of CD28 co-stimulation (81, 82). A “cell-intrinsic” mechanism of CTLA-4–mediated modulation of CD28 has been also described (Fig. 4) and is based on a direct competition for CD80 binding. High levels of CTLA-4 selectively impair CD80–CD28 interaction, due to the higher affinity of CD80 for CTLA-4 than CD28. Consequently, in cells expressing high levels of CTLA-4, such as Treg cells, CD80 is preferentially engaged by CTLA-4 and therefore CD86 becomes the main ligand available for CD28 co-stimulation, despite its lower affinity for this molecule. CD86-CD28 binding supports Treg cell proliferation and regulatory function by maintaining high expression of suppressive markers such as CTLA-4, ICOS, and OX40 (64). Consistently, CD80–CD28 interaction can be rescued in CTLA-4–deficient Treg cells, resulting in Treg cell expansion with impaired suppressive function (83, 84).Therefore, CD86 represents the preferential CD28 ligand selectively required for Treg proliferation, homeostasis, and activation.
Figure 4.
Cell intrinsic and extrinsic regulation of T cell activation.A, high levels of CTLA-4 expression on Treg cell surface selectively inhibit the CD80–CD28 interaction as CTLA-4, by binding to CD80, prevents its binding to CD28, thus leaving the molecule CD86 as the only available ligand for CD28. In addition, the CD28/CD86 interaction further supports the suppressive phenotype of Treg cells by inducing the expression of suppressive markers (including CTLA-4 itself). B, Treg cells can inhibit T cell activation through a mechanism of CTLA-4–mediated trans-endocytosis of CD80 and/or CD86 from the APC surface, thereby preventing co-stimulation of CD28 on the T cell surface, thus inhibiting T cell activation. APC, antigen presenting cell; Treg, regulatory T cell.
However, the preference for CTLA-4 or CD28 binding and, consequently, the activation of downstream signaling is not only due to CD80 and CD86 levels but also to their different expression patterns on APCs. Indeed, CD86 is constitutively expressed on APC, whereas CD80 is mainly upregulated upon activation, and a differential cell-specific expression of these two molecules exists, with possible implications for preferential activation of either CD28 or CTLA-4 pathway (62, 63). In addition to the direct competition with CD28 for the shared ligands, CTLA-4 also interferes with the translocation of CD28 to the so called “immunological synapse” (IS), a specialized junction at the T cell–APC interface, crucial for the functional maturation and activation of T cells (85, 86). The IS consists of clusters of TCR-associated receptors and their downstream signaling molecules, in which TCR signals converge to initiate T cell responses. The IS includes a central supramolecular activation cluster (cSMAC), where the co-stimulatory signal originates and is finely regulated by competition between CD28 and CTLA-4. More specifically, CD28-mediated T cell co-stimulation occurs in a TCR-CD3 low-density region (CD3lo) of cSMAC, following specific recruitment of protein kinase C-q (PKCq). In this context, CTLA-4 negatively regulates T cell activation through a dynamic mechanism in which it translocates from the secretory granules to the IS upon TCR engagement, accumulates in the CD3lo region of the cSMAC, and inhibits CD28 and PKCq recruitment. This mechanism has been observed both in Tconv and Treg cells, in which the constitutive expression of CTLA-4 results in the lack of PKCq translocation to the cSMAC, thus contributing to Treg cell in vitro anergic state (61, 86). Although several mechanisms have been proposed, how and whether CTLA-4 can exert its regulatory function at the molecular level remains still debated. However, it is now well accepted that CTLA-4 represents an essential checkpoint molecule for the control of autoimmunity, mainly because of its contribution to Treg cell generation and acquisition of suppressive function, which require a balanced integration of inhibitory and co-stimulatory signals coordinated by the CTLA-4–CD28 pathway.
In conclusion, given the relevance of CTLA-4–CD28 pathway in the regulation of TCR signaling, a precise understanding of its impact on immune system function may provide important insights on whether and how its modulation can suppress autoimmunity and promote anti-tumor immune response.
Multifunctional roles for IL-2/IL-2R signaling in T cell tolerance
One of the main cytokines which supports TCR signaling in promoting Treg cell generation is IL-2, which was first identified in 1976 as a crucial T cell growth factor, exerting a wide range of actions, including regulation of cell survival, proliferation, differentiation, and acquisition of T cell effector functions (87, 88).
IL-2 is mainly produced by activated T cells after engagement of the TCR and the costimulatory molecule CD28 and mediates its biological functions through the binding to its high affinity receptor (IL-2R) which consists of three subunits: the α-chain (IL-2Rα, also known as CD25), the β-chain (IL-2Rβ, also known as CD122), whose expression is dependent by TCR-signaling, and the common cytokine receptor γ-chain (γc, also known as CD132), which is constitutively expressed by T cells (89, 90, 91). The IL-2/IL-2R complex activates downstream signaling pathways through the association of the tyrosine kinases JAK1 and JAK3 with the cytoplasmic tails of IL-2Rβ and γc, leading to JAK cross-phosphorylation and recruitment of members of the STATs family (92, 93, 94). IL-2 is able to activate several members of the STAT family, including STAT1, STAT3, and STAT5, which is the predominant IL-2 signaling–associated molecule (95). Upon recruitment, two STAT5 isoforms (STAT5A and STAT5B) are activated via JAK-mediated phosphorylation of the tyrosine 694 (STAT5A) and 699 (STAT5B) residues, which induces their dimerization and nuclear translocation, with consequent activation of their transcriptional activity, elicited through their binding to DNA or recruitment of other co-factors (96). In addition to tyrosine 694/699, a number of serine residues within STAT5A and STAT5B have been also identified as phosphorylation sites, including S127/128 (STAT5A), S725 (STAT5A), S730 (STAT5B), and S780 (STAT5A). While IL-2–dependent phosphorylation of these sites has been suggested to modulate STAT5 transcriptional activity, their precise functions and roles in T cell regulation are still under investigation (97, 98, 99). Although STAT5 is a critical downstream mediator of IL-2 signaling, constitutively active STAT5 is not sufficient per se to reproduce all the IL-2 biological effects, as IL-2–mediated pathways include other signaling cascades, such as Raf-ERK MAP kinases, PI3K-Akt, and mTOR (87, 97, 100, 101).
The dynamic ability of IL-2 to either promote or repress specific T helper cell programs depends on several mechanisms, including modulation of both cytokines and cytokine receptors expression, as well as negative regulation of cell type–specific transcription factors with activation of precise metabolic programs. In this regard, IL-2R–mediated signaling display a key role in the control of both effector T and Treg cell responses (102, 103, 104). Indeed, IL-2 contributes to optimal clonal expansion of activated T cells, driving their terminal differentiation and controlling T cell memory development and survival (91). IL-2 is also critical for Treg cell development, homeostasis, proliferation, and maintenance in the peripheral tissues, as it has been shown to sustain Foxp3 expression, the key transcription factor defining Treg cell identity and required for their function (105, 106, 107), despite contrasting data from Fontenot et al. have shown that IL-2 signaling is dispensable for the induction of Foxp3 expression in thymocytes (87, 106). All these IL-2–mediated effects on Treg cells are also due to their constitutive CD25 expression, which allows them to respond to low doses of IL-2 and sustains Treg cell function and CTLA-4 expression (91, 107). The process of tTreg cell generation is controlled by a tight network of relationship among TCR-mediated signal, IL-2R expression, and IL-2-mediated Foxp3 induction; specifically, during thymic Treg cell development, in a first cytokine-independent step, TCR- and CD28-mediated signaling generates IL-2–responsive Treg cell progenitors lacking Foxp3 expression but characterized by CD25 upregulation (Fig. 5). This high CD25 expression makes the precursors competitive for IL-2 uptake, which directly induces Foxp3 expression and finalizes Treg cell development. In the second step, in a TCR-independent but IL-2/STAT5–dependent process, IL2 mediates the rapid conversion of these Treg cell precursors into mature Foxp3+Treg cells (108, 109). Gomez-Rodriguez et al. suggested a possible mechanism by which IL-2 may regulate TCR signaling during Treg cell induction. Indeed, the authors showed that the IL-2–inducible T-cell kinase ITK mediated the cross-talk between TCR and IL-2 signaling, thereby influencing the balance between Th17 and Treg cells (52). Although Treg cells require TCR signaling to proliferate, in the presence of elevated IL-2 levels or when STAT5 is selectively activated in Treg cells, their division can occur independently of MHC class II and TCR signaling (110).
Figure 5.
Two-step model promoting Foxp3+Treg cell differentiation. Thymic Treg cell development involves a two-step process: the first step is cytokine-independent and is induced by strong TCR stimulation occurring in developing CD4-single positive thymocytes (blue cell). This phenomenon leads to upregulation of α-chain of the high affinity IL-2 receptor (CD25), generating CD4+CD25+ Treg cell precursors (orange cell), which do not express Foxp3. The second cytokine-dependent step results in the upregulation of Foxp3, driving the conversion of Treg cell precursors into mature Treg cells (green cell). TCR, T cell receptor; Treg, regulatory T cell.
Several studies have shown that IL-2, together with transforming growth factor (TGF)-β, is an essential polarizing cytokine involved in inducible Treg cell differentiation, as its neutralization abrogated this process, even in the presence of TGF-β (111, 112, 113). Mechanistically, IL-2 has been shown to control the stability of Foxp3 expression in TGF-β–induced Treg cells in vivo, by promoting the demethylation of the conserved non-coding sequence 2 region (also known as Treg cell–specific demethylated region or TSDR) within the Foxp3 gene (114). As we previously reported, STAT5 is considered a critical mediator of IL-2 signaling: specifically, its deficiency is associated with impaired Treg cell generation and its transient activation has proven to be sufficient to increase Treg cell number even in IL-2–deficient mice (115, 116). In addition, mice over-expressing a constitutively active form of STAT5B are protected from graft-versus-host disease and display increased Treg cell number with enhanced suppressive function, as compared to WT mice (117, 118). Supporting these observations, STAT5B knocking down in human primary T cells results in reduced Foxp3 expression; indeed a human rare autosomal recessive disease, characterized by STAT5B deficiency, has been associated with chronic lung disease, autoimmunity, and impaired Treg cell number and function (119) (Fig. 1).
The dysregulation of IL-2–mediated pathways has been identified as a key feature in the pathogenesis of several autoimmune disorders, resulting in a reduced number and altered function of Treg cells (Fig. 1). This concept is supported by observations showing that polymorphisms altering IL-2 signaling in humans are associated with autoimmune diseases, including type 1 diabetes, celiac disease, multiple sclerosis (MS), Graves’ disease, Sjögren’s syndrome, and rheumatoid arthritis (120, 121, 122, 123). Specifically, in subjects with type 1 diabetes, IL-2RA polymorphisms are associated with reduced soluble (s)IL-2R levels and lower STAT5A phosphorylation. Also, the diabetes-associated IL-2RA haplotype (rs12722495) correlates with reduced IL-2 responsiveness, impaired Foxp3 expression, and altered Treg cell suppressive function (124, 125). Overlapping results have been also obtained in MS, in which the rs2104286 polymorphism in the IL-2RA locus has been associated with a higher risk of developing the disease and with increased expression of IL-2Rα on naïve Th cells, which preferentially differentiate into encephalitogenic granulocyte-macrophage colony-stimulating factor–producing effector Th cells (121, 126).
Finally, IL-2 has been shown to play a critical role also in sensitizing T cells to antigen-induced cell death (AICD), one of the mechanisms responsible for the peripheral deletion of autoreactive T cells and induction of peripheral tolerance (127). Indeed, although IL-2 is an important T cell growth and survival factor, it can also limit the magnitude of T cell response, by inducing the upregulation of Fas, its ligand (FasL), and tumor necrosis factor receptor expression and concomitantly downregulating the cysteine aspartic enzyme inhibition factor (C-FLIP), thereby promoting AICD. In support of this evidence, KO mice for IL-2 or IL-2Rα are not sensitive to T cell AICD (127) (Fig. 1). In addition, IL-2 has been shown to promote the synthesis of interferon (IFN)-γ, which is a critical cytokine for AICD (128). Specifically, Refaeli et al. found that CD4+ T cells lacking IFN-γ or the STAT1 transcription factor were resistant to AICD, as IFN-γ was required for caspase-8 production, involved in cell death induction. On the contrary, forced expression of caspase-8 was able to restore T cell sensitivity to AICD in STAT1-deficient mice (129).
All these data support the notion that IL-2 regulates T cell differentiation upon antigen stimulation and represents a critical driver of T cell fate decision.
Role of CD45 in the modulation of TCR signaling and immune tolerance
CD45, the leukocyte common antigen, is a transmembrane tyrosine phosphatase expressed on all nucleated hematopoietic cells. It plays a key role in controlling T lymphocyte activation, cytokine production, and the thymic development by regulating TCR-mediated signaling. In this context, CD45 can both positively and negatively regulate T cell activation; indeed, it is involved in the activation of the Src family kinase LCK but paradoxically, it has also been implicated in the suppression of the TCR-activated pathway, by dephosphorylating signaling motifs within the TCR complex (130, 131, 132). CD45 has been shown to be differentially required for the development and function of B and T lymphocytes; indeed, the development of B cells appeared normal in mice homozygous for the CD45-exon6 mutation, whereas thymocyte maturation was blocked at the transition stage from immature CD4+CD8+ to mature CD4+ or CD8+ cells (133).
This phosphatase can also act as a negative regulator of cytokine receptor signaling; in particular, targeted inhibition of CD45 has been shown to associate with enhanced cytokine and IFN receptor–mediated activation of JAKs and STAT proteins (134). Like many other protein tyrosine phosphatases, CD45 consists of an extracellular receptor-like region and an N-terminal region accounting for different isoform variants (135, 136). Indeed, multiple isoforms are generated by a tightly regulated alternative splicing mechanism, involving specific exons, in particular exons 4, 5, and 6 and resulting in differential inclusion of segments A, B, and C, respectively (137). The distinct isoforms differ in the size of their extracellular domains, and T cells can express the high or low molecular weight form of CD45 (CD45RA or CD45RO, respectively), depending on their activation state; indeed, differentiation of naive T cells, which express different larger isoforms (including RABC, RAB, RBC, and RB), into memory T cells is accompanied by exon exclusion for the production of the short isoform RO with concomitant downregulation of CD45RB expression. Basadonna et al. have shown that anti-CD45RB treatment can induce long-term engraftment of transplanted islets in diabetic mice, with reduced insulitis and preserved islet integrity (138, 139). Specifically, this treatment resulted in a shift in CD45 isoform expression on T cells from higher to lower molecular weight isoforms (CD45RO), together with a shift from CD45RBHi to CD45RBLo. These phenomena were also associated with increased expression of Th2-type cytokines, such as IL-4 and IL-10, suggesting that the change in CD45 isoforms induced by mAb treatment can cause a shift in the functional repertoire of responding T cells, skewing the immune response towards allograft tolerance (140). In another paper by the same group, it has been demonstrated that the beneficial effect of anti-CD45RB treatment on allograft survival during transplantation can act through mechanisms involving CTLA-4 upregulation, as its inhibition reverted the phenomenon, thus demonstrating a direct link between CD45 and CTLA-4 (141).
In addition to the regulation of CTLA-4 expression, CD45 targeting can also control immune tolerance through the expansion of Treg cells; indeed, treatment with a tolerogenic anti-CD45RB mAb has been shown to increase homeostatic Treg cell proliferation in WT mice. Moreover, it has been also shown that CD45 ligation prolonged the interaction between Treg cells and DCs in vivo, reducing Treg cell motility and promoting nuclear translocation of nuclear factor of activated T cells, which is required for Treg cell proliferation. These data suggest that peripheral Treg homeostasis can be controlled in vivo through the specific targeting of CD45, which in turn promotes the interaction of Treg cells with DCs (142).
CD45 is also involved in the homeostatic control of T cell proliferation; in particular, van Vliet et al. have shown that the interaction between CD45 expressed by effector T cells with the C-type lectin receptor MGL expressed by APCs inhibits TCR-mediated signaling and cytokine response, resulting in decreased T cell proliferation and increased T cell death, thus preventing tissue damage and autoimmunity (143).
Recent experimental evidence has shown that a heterozygous C→G transversion at nucleotide 77 in exon 4/A of the gene encoding CD45 results in a defective CD45 alternative splicing, with altered expression of its isoforms, which has been associated with increased susceptibility to MS (144, 145) (Fig. 1). The same mutation has also been studied in the context of other autoimmune diseases; indeed, a significantly higher frequency of this mutation has been observed in patients with autoimmune hepatitis, characterized by the loss of tolerance against liver resident antigens (146). Furthermore, altered expression of the different isoforms of CD45 within CD4+ T cell subset has also been reported in children with infantile cholestasis and in patients with systemic lupus erythematosus (147, 148, 149).
In conclusion, while it is clear that CD45 can act as both a positive and negative regulator of T cell activation, several issues require further clarification, such as the functional role of the different CD45 isoforms and the regulation of their expression and alternative splicing. Understanding these mechanisms may be useful for the development of novel therapeutic tools able to modulate and control CD45 activity to direct T cell activation towards a pro-inflammatory or tolerogenic profile.
Concluding remarks
TCR-mediated signaling plays a key role in the control of several mechanisms related to T cell biology, including their development, activation, differentiation, proliferation, and the acquisition of effector functions. Therefore, TCR-mediated pathways need to be finely-tuned to prevent the development of pathological conditions. In this context, positive and negative mechanisms of T cell activation are able to integrate signals from peptide:MHC complexes and cell surface accessory molecules, translating them into intracellular events that influence cell fate decisions. In the past few years, compelling experimental evidence has demonstrated that TCR signal strength can regulate CD4+ T cell differentiation also controlling the phenotype and effector functions of Th1/Th17 cells or Treg cells. In determining the precise differentiative cell fate, the activity of TCR signaling can control T cell activation or tolerance induction toward a particular antigen. In this context, both T cell hypo- and hyper-responsiveness, caused by abnormalities in TCR-signaling pathways, can lead to the development of autoimmunity. In light of all these considerations, mechanisms or molecules able to modify TCR-mediated signaling and its regulatory pathways might represent a potential therapeutic target to control T cell activation and possibly modulate their fate to drive the immune response towards an inflammatory or tolerogenic phenotype. In conclusion, further studies are needed to fully understand these regulatory mechanisms, which could allow us to modulate immune responses for the treatment of immune-mediated diseases, such as immunodeficiency or autoimmunity.
Conflict of interest
The authors declare that they have no conflicts of interests with the contents of this article.
Acknowledgments
Author contributions
F. C., C. P., and G. M. conceptualization; F. C., C. R., A. C., C. L. R., C. F., A. M., C. P., and G. M. writing–original draft; F. C., C. R., A. C., C. L. R., C. F., A. M., C. P., and G. M. writing–review and editing.
Funding and additional information
This work was supported by the Italian Ministry of University and Research (MUR) (Bando PRIN 2022 Prot. 2022LNHZAP) and MUR PNRR Extended Partnership (INF-ACT no. PE00000007 and MNESYS no. PE00000006) and the Italian Ministry of Health (Bando Ricerca Finalizzata 2019 RF-2019-12371111 and Bando PNRR 2022 PNRR-MAD-2022-12375634) to G. M.; the Italian Ministry of Health (grant GR-2018-12366154), Fondazione Italiana Sclerosi Multipla (FISM no. 2022-PRsingle/013), and the Italian Ministry of University and Research (MUR) bando PRIN 2022 PNRR (grant n. P2022T4PKT) to C. P.; the Italian Ministry of Health, Bando PNRR 2022 (PNRR-MAD-2022-12376126) to C. P. and F. C.; the Italian Ministry of Health, Bando Ricerca Finalizzata 2021 (grant n. GR-2021-12373337) and the Italian Ministry of University and Research (MUR) Bando PRIN 2022 (grant n. 2022YMJXYT) and bando PRIN 2022 PNRR (grant n. P2022CMK43) to F. C.; the Italian Ministry of University and Research (MUR) Bando PRIN 2022 (grant n. 20225KH7BZ) to C. L. R. A. M. is recipient of a fellowship in General Surgery at University of Naples “Federico II”. Servier Medical Art provided some of the illustrations used to create the figure (http://www.servier.fr/servier-medical-art).
Reviewed by members of the JBC Editorial Board. Edited by Alex Toker
Contributor Information
Claudio Procaccini, Email: claudio.procaccini@cnr.it.
Giuseppe Matarese, Email: giuseppe.matarese@unina.it.
References
- 1.Chen L., Flies D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 3.Hwang J.R., Byeon Y., Kim D., Park S.G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 2020;52:750–761. doi: 10.1038/s12276-020-0435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acuto O., Di Bartolo V., Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat. Rev. Immunol. 2008;8:699–712. doi: 10.1038/nri2397. [DOI] [PubMed] [Google Scholar]
- 5.Stone J.D., Chervin A.S., Kranz D.M. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126:165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan W., Cao Y.M., Zhao X., Schrom E.C., Jia D., Song J., et al. TCR ligand potency differentially impacts PD-1 inhibitory effects on diverse signaling pathways. J. Exp. Med. 2023;220 doi: 10.1084/jem.20231242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Josefowicz S.Z., Lu L.F., Rudensky A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S., Svensson M.N.D., Harder N.H.O., Hsieh W.C., Santelli E., Kiosses W.B., et al. PTPN22 phosphorylation acts as a molecular rheostat for the inhibition of TCR signaling. Sci. Signal. 2020;13 doi: 10.1126/scisignal.aaw8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vang T., Congia M., Macis M.D., Musumeci L., Orrú V., Zavattari P., et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat. Genet. 2005;37:1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 10.Pao L.I., Badour K., Siminovitch K.A., Neel B.G. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu. Rev. Immunol. 2007;25:473–523. doi: 10.1146/annurev.immunol.23.021704.115647. [DOI] [PubMed] [Google Scholar]
- 11.Feinerman O., Germain R.N., Altan-Bonnet G. Quantitative challenges in understanding ligand discrimination by alphabeta T cells. Mol. Immunol. 2008;45:619–631. doi: 10.1016/j.molimm.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kersh G.J., Kersh E.N., Fremont D.H., Allen P.M. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity. 1998;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 13.George A.J., Stark J., Chan C. Understanding specificity and sensitivity of T-cell recognition. Trends Immunol. 2005;26:653–659. doi: 10.1016/j.it.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Bhakta N.R., Oh D.Y., Lewis R.S. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat. Immunol. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 15.Daniels M.A., Teixeiro E., Gill J., Hausmann B., Roubaty D., Holmberg K., et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 16.Sadegh-Nasseri S., Dalai S.K., Korb Ferris L.C., Mirshahidi S. Suboptimal engagement of the T-cell receptor by a variety of peptide-MHC ligands triggers T-cell anergy. Immunology. 2010;129:1–7. doi: 10.1111/j.1365-2567.2009.03206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan M.S., Boesteanu A., Reed A.J., Petrone A.L., Holenbeck A.E., Lerman M.A., et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 18.Lee H.M., Bautista J.L., Scott-Browne J., Mohan J.F., Hsieh C.S. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity. 2012;37:475–486. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran A.E., Holzapfel K.L., Xing Y., Cunningham N.R., Maltzman J.S., Punt J., et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cozzo Picca C., Simons D.M., Oh S., Aitken M., Perng O.A., Mergenthaler C., et al. CD4⁺CD25⁺Foxp3⁺ regulatory T cell formation requires more specific recognition of a self-peptide than thymocyte deletion. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14890–14895. doi: 10.1073/pnas.1103810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprouse M.L., Scavuzzo M.A., Blum S., Shevchenko I., Lee T., Makedonas G., et al. High self-reactivity drives T-bet and potentiates Treg function in tissue-specific autoimmunity. JCI Insight. 2018;3 doi: 10.1172/jci.insight.97322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprouse M.L., Shevchenko I., Scavuzzo M.A., Joseph F., Lee T., Blum S., et al. Cutting edge: low-affinity TCRs support regulatory T cell function in autoimmunity. J. Immunol. 2018;200:909–914. doi: 10.4049/jimmunol.1700156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Hagan K.L., Choi J., Pryshchep O., Chernoff J., Phee H. Pak2 links TCR signaling strength to the development of regulatory T cells and maintains peripheral tolerance. J. Immunol. 2015;195:1564–1577. doi: 10.4049/jimmunol.1500843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mongellaz C., Vicente R., Noroski L.M., Noraz N., Courgnaud V., Chinen J., et al. Combined immunodeficiency caused by pathogenic variants in the ZAP70 C-terminal SH2 domain. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1155883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaguchi N., Takahashi T., Hata H., Nomura T., Tagami T., Yamazaki S., et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka S., Maeda S., Hashimoto M., Fujimori C., Ito Y., Teradaira S., et al. Graded attenuation of TCR signaling elicits distinct autoimmune diseases by altering thymic T cell selection and regulatory T cell function. J. Immunol. 2010;185:2295–2305. doi: 10.4049/jimmunol.1000848. [DOI] [PubMed] [Google Scholar]
- 27.Hsu L.Y., Tan Y.X., Xiao Z., Malissen M., Weiss A. A hypomorphic allele of ZAP-70 reveals a distinct thymic threshold for autoimmune disease versus autoimmune reactivity. J. Exp. Med. 2009;206:2527–2541. doi: 10.1084/jem.20082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cauwe B., Tian L., Franckaert D., Pierson W., Staats K.A., Schlenner S.M., et al. A novel Zap70 mutation with reduced protein stability demonstrates the rate-limiting threshold for Zap70 in T-cell receptor signalling. Immunology. 2014;141:377–387. doi: 10.1111/imm.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siggs O.M., Miosge L.A., Yates A.L., Kucharska E.M., Sheahan D., Brdicka T., et al. Opposing functions of the T cell receptor kinase ZAP-70 in immunity and tolerance differentially titrate in response to nucleotide substitutions. Immunity. 2007;27:912–926. doi: 10.1016/j.immuni.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka A., Maeda S., Nomura T., Llamas-Covarrubias M.A., Tanaka S., Jin L., et al. Construction of a T cell receptor signaling range for spontaneous development of autoimmune disease. J. Exp. Med. 2023;220 doi: 10.1084/jem.20220386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Au-Yeung B.B., Levin S.E., Zhang C., Hsu L.Y., Cheng D.A., Killeen N., et al. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat. Immunol. 2010;11:1085–1092. doi: 10.1038/ni.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lui V.G., Hoenig M., Cabrera-Martinez B., Baxter R.M., Garcia-Perez J.E., Bailey O., et al. A partial human LCK defect causes a T cell immunodeficiency with intestinal inflammation. J. Exp. Med. 2024;221 doi: 10.1084/jem.20230927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koonpaew S., Shen S., Flowers L., Zhang W. LAT-mediated signaling in CD4+CD25+ regulatory T cell development. J. Exp. Med. 2006;203:119–129. doi: 10.1084/jem.20050903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuck M.I., Zhu M., Shen S., Zhang W. The role of the LAT-PLC-gamma1 interaction in T regulatory cell function. J. Immunol. 2010;184:2476–2486. doi: 10.4049/jimmunol.0902876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu G., Chen Y., Yu M., Podd A., Schuman J., He Y., et al. Phospholipase C{gamma}1 is essential for T cell development, activation, and tolerance. J. Exp. Med. 2010;207:309–318. doi: 10.1084/jem.20090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt A.M., Lu W., Sindhava V.J., Huang Y., Burkhardt J.K., Yang E., et al. Regulatory T cells require TCR signaling for their suppressive function. J. Immunol. 2015;194:4362–4370. doi: 10.4049/jimmunol.1402384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter J.D., Calabrese G.M., Naganuma M., Lorenz U. Deficiency of the Src homology region 2 domain-containing phosphatase 1 (SHP-1) causes enrichment of CD4+CD25+ regulatory T cells. J. Immunol. 2005;174:6627–6638. doi: 10.4049/jimmunol.174.11.6627. [DOI] [PubMed] [Google Scholar]
- 38.Ordoñez-Rueda D., Lozano F., Sarukhan A., Raman C., Garcia-Zepeda E.A., Soldevila G. Increased numbers of thymic and peripheral CD4+ CD25+Foxp3+ cells in the absence of CD5 signaling. Eur. J. Immunol. 2009;39:2233–2247. doi: 10.1002/eji.200839053. [DOI] [PubMed] [Google Scholar]
- 39.Hwang S., Song K.D., Lesourne R., Lee J., Pinkhasov J., Li L., et al. Reduced TCR signaling potential impairs negative selection but does not result in autoimmune disease. J. Exp. Med. 2012;209:1781–1795. doi: 10.1084/jem.20120058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M.O., Rudensky A.Y. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 2016;16:220–233. doi: 10.1038/nri.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouyang W., Li M.O. Foxo: in command of T lymphocyte homeostasis and tolerance. Trends Immunol. 2011;32:26–33. doi: 10.1016/j.it.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jun J.E., Kulhanek K.R., Chen H., Chakraborty A., Roose J.P. Alternative ZAP70-p38 signals prime a classical p38 pathway through LAT and SOS to support regulatory T cell differentiation. Sci. Signal. 2019;12 doi: 10.1126/scisignal.aao0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahmud S.A., Manlove L.S., Schmitz H.M., Xing Y., Wang Y., Owen D.L., et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat. Immunol. 2014;15:473–481. doi: 10.1038/ni.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattacharyya N.D., Feng C.G. Regulation of T Helper cell fate by TCR signal strength. Front Immunol. 2020;11:624. doi: 10.3389/fimmu.2020.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine A.G., Arvey A., Jin W., Rudensky A.Y. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vahl J.C., Drees C., Heger K., Heink S., Fischer J.C., Nedjic J., et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Gottschalk R.A., Corse E., Allison J.P. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J. Exp. Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apostolou I., von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miskov-Zivanov N., Turner M.S., Kane L.P., Morel P.A., Faeder J.R. The duration of T cell stimulation is a critical determinant of cell fate and plasticity. Sci. Signal. 2013;6:ra97. doi: 10.1126/scisignal.2004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kretschmer K., Apostolou I., Hawiger D., Khazaie K., Nussenzweig M.C., von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 51.Josefowicz S.Z., Wilson C.B., Rudensky A.Y. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J. Immunol. 2009;182:6648–6652. doi: 10.4049/jimmunol.0803320. [DOI] [PubMed] [Google Scholar]
- 52.Gomez-Rodriguez J., Wohlfert E.A., Handon R., Meylan F., Wu J.Z., Anderson S.M., et al. Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J. Exp. Med. 2014;211:529–543. doi: 10.1084/jem.20131459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powell J.D., Pollizzi K.N., Heikamp E.B., Horton M.R. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Procaccini C., De Rosa V., Galgani M., Abanni L., Calì G., Porcellini A., et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–941. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hawse W.F., Boggess W.C., Morel P.A. TCR signal strength regulates Akt substrate specificity to induce alternate Murine Th and T regulatory cell differentiation programs. J. Immunol. 2017;199:589–597. doi: 10.4049/jimmunol.1700369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauer S., Bruno L., Hertweck A., Finlay D., Leleu M., Spivakov M., et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner M.S., Kane L.P., Morel P.A. Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J. Immunol. 2009;183:4895–4903. doi: 10.4049/jimmunol.0901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xia S., Chen Q., Niu B. CD28: a new drug target for immune disease. Curr. Drug Targets. 2020;21:589–598. doi: 10.2174/1389450120666191114102830. [DOI] [PubMed] [Google Scholar]
- 59.Brown C.C., Rudensky A.Y. Spatiotemporal regulation of peripheral T cell tolerance. Science. 2023;380:472–478. doi: 10.1126/science.adg6425. [DOI] [PubMed] [Google Scholar]
- 60.ElTanbouly M.A., Noelle R.J. Rethinking peripheral T cell tolerance: checkpoints across a T cell's journey. Nat. Rev. Immunol. 2021;21:257–267. doi: 10.1038/s41577-020-00454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokosuka T., Kobayashi W., Takamatsu M., Sakata-Sogawa K., Zeng H., Hashimoto-Tane A., et al. Spatiotemporal basis of CTLA-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity. 2010;33:326–339. doi: 10.1016/j.immuni.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Ganesan A., Moon T.C., Barakat K.H. Revealing the atomistic details behind the binding of B7-1 to CD28 and CTLA-4: a comprehensive protein-protein modelling study. Biochim. Biophys. Acta Gen. Subj. 2018;1862:2764–2778. doi: 10.1016/j.bbagen.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 63.Van Coillie S., Wiernicki B., Xu J. Molecular and cellular functions of CTLA-4. Adv. Exp. Med. Biol. 2020;1248:7–32. doi: 10.1007/978-981-15-3266-5_2. [DOI] [PubMed] [Google Scholar]
- 64.Halliday N., Williams C., Kennedy A., Waters E., Pesenacker A.M., Soskic B., et al. CD86 is a selective CD28 ligand supporting FoxP3+ regulatory T cell homeostasis in the presence of high levels of CTLA-4. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.600000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Punt J.A., Osborne B.A., Takahama Y., Sharrow S.O., Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J. Exp. Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amsen D., Kruisbeek A.M. CD28-B7 interactions function to co-stimulate clonal deletion of double-positive thymocytes. Int. Immunol. 1996;8:1927–1936. doi: 10.1093/intimm/8.12.1927. [DOI] [PubMed] [Google Scholar]
- 67.Kishimoto H., Sprent J. Several different cell surface molecules control negative selection of medullary thymocytes. J. Exp. Med. 1999;190:65–73. doi: 10.1084/jem.190.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li R., Page D.M. Requirement for a complex array of costimulators in the negative selection of autoreactive thymocytes in vivo. J. Immunol. 2001;166:6050–6056. doi: 10.4049/jimmunol.166.10.6050. [DOI] [PubMed] [Google Scholar]
- 69.Tan R., Teh S.J., Ledbetter J.A., Linsley P.S., Teh H.S. B7 costimulates proliferation of CD4-8+ T lymphocytes but is not required for the deletion of immature CD4+8+ thymocytes. J. Immunol. 1992;149:3217–3224. [PubMed] [Google Scholar]
- 70.Walunas T.L., Sperling A.I., Khattri R., Thompson C.B., Bluestone J.A. CD28 expression is not essential for positive and negative selection of thymocytes or peripheral T cell tolerance. J. Immunol. 1996;156:1006–1013. [PubMed] [Google Scholar]
- 71.Hinterberger M., Wirnsberger G., Klein L. B7/CD28 in central tolerance: costimulation promotes maturation of regulatory T cell precursors and prevents their clonal deletion. Front Immunol. 2011;2:30. doi: 10.3389/fimmu.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hogquist K.A., Jameson S.C. The self-obsession of T cells: how TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat. Immunol. 2014;15:815–823. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verhagen J., Genolet R., Britton G.J., Stevenson B.J., Sabatos-Peyton C.A., Dyson J., et al. CTLA-4 controls the thymic development of both conventional and regulatory T cells through modulation of the TCR repertoire. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E221–E230. doi: 10.1073/pnas.1208573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riella L.V., Liu T., Yang J., Chock S., Shimizu T., Mfarrej B., et al. Deleterious effect of CTLA4-Ig on a Treg-dependent transplant model. Am. J. Transplant. 2012;12:846–855. doi: 10.1111/j.1600-6143.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 75.Salomon B., Lenschow D.J., Rhee L., Ashourian N., Singh B., Sharpe A., et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 76.Zhang R., Huynh A., Whitcher G., Chang J., Maltzman J.S., Turka L.A. An obligate cell-intrinsic function for CD28 in Tregs. J. Clin. Invest. 2013;123:580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmidt E.M., Wang C.J., Ryan G.A., Clough L.E., Qureshi O.S., Goodall M., et al. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J. Immunol. 2009;182:274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- 78.Waterhouse P., Penninger J.M., Timms E., Wakeham A., Shahinian A., Lee K.P., et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 79.Edner N.M., Carlesso G., Rush J.S., Walker L.S.K. Targeting co-stimulatory molecules in autoimmune disease. Nat. Rev. Drug Discov. 2020;19:860–883. doi: 10.1038/s41573-020-0081-9. [DOI] [PubMed] [Google Scholar]
- 80.Hosseini A., Gharibi T., Marofi F., Babaloo Z., Baradaran B. CTLA-4: from mechanism to autoimmune therapy. Int. Immunopharmacol. 2020;80 doi: 10.1016/j.intimp.2020.106221. [DOI] [PubMed] [Google Scholar]
- 81.Qureshi O.S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E.M., et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ovcinnikovs V., Ross E.M., Petersone L., Edner N.M., Heuts F., Ntavli E., et al. CTLA-4-mediated transendocytosis of costimulatory molecules primarily targets migratory dendritic cells. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aaw0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klocke K., Sakaguchi S., Holmdahl R., Wing K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E2383–E2392. doi: 10.1073/pnas.1603892113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schubert D., Bode C., Kenefeck R., Hou T.Z., Wing J.B., Kennedy A., et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 2014;20:1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grakoui A., Bromley S.K., Sumen C., Davis M.M., Shaw A.S., Allen P.M., et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 86.Finetti F., Baldari C.T. The immunological synapse as a pharmacological target. Pharmacol. Res. 2018;134:118–133. doi: 10.1016/j.phrs.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 87.Ross S.H., Cantrell D.A. Signaling and function of interleukin-2 in T lymphocytes. Annu. Rev. Immunol. 2018;36:411–433. doi: 10.1146/annurev-immunol-042617-053352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., et al. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983;302:305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- 89.Spolski R., Li P., Leonard W.J. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018;18:648–659. doi: 10.1038/s41577-018-0046-y. [DOI] [PubMed] [Google Scholar]
- 90.Johnson K., Choi Y., Wu Z., Ciardelli T., Granzow R., Whalen C., et al. Soluble IL-2 receptor beta and gamma subunits: ligand binding and cooperativity. Eur. Cytokine Netw. 1994;5:23–34. [PubMed] [Google Scholar]
- 91.Malek T.R. The biology of interleukin-2. Annu. Rev. Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 92.Li Y., Li X., Geng X., Zhao H. The IL-2A receptor pathway and its role in lymphocyte differentiation and function. Cytokine Growth Factor Rev. 2022;67:66–79. doi: 10.1016/j.cytogfr.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 93.Josefowicz S.Z. Regulators of chromatin state and transcription in CD4 T-cell polarization. Immunology. 2013;139:299–308. doi: 10.1111/imm.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soufi A., Donahue G., Zaret K.S. Facilitators and impediments of the pluripotency reprogramming factors' initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hou J., Schindler U., Henzel W.J., Wong S.C., McKnight S.L. Identification and purification of human Stat proteins activated in response to interleukin-2. Immunity. 1995;2:321–329. doi: 10.1016/1074-7613(95)90140-x. [DOI] [PubMed] [Google Scholar]
- 96.Moriggl R., Sexl V., Piekorz R., Topham D., Ihle J.N. Stat5 activation is uniquely associated with cytokine signaling in peripheral T cells. Immunity. 1999;11:225–230. doi: 10.1016/s1074-7613(00)80097-7. [DOI] [PubMed] [Google Scholar]
- 97.Jones D.M., Read K.A., Oestreich K.J. Dynamic roles for IL-2-STAT5 signaling in effector and regulatory CD4(+) T cell populations. J. Immunol. 2020;205:1721–1730. doi: 10.4049/jimmunol.2000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beadling C., Ng J., Babbage J.W., Cantrell D.A. Interleukin-2 activation of STAT5 requires the convergent action of tyrosine kinases and a serine/threonine kinase pathway distinct from the Raf1/ERK2 MAP kinase pathway. EMBO J. 1996;15:1902–1913. [PMC free article] [PubMed] [Google Scholar]
- 99.Beuvink I., Hess D., Flotow H., Hofsteenge J., Groner B., Hynes N.E. Stat5a serine phosphorylation. Serine 779 is constitutively phosphorylated in the mammary gland, and serine 725 phosphorylation influences prolactin-stimulated in vitro DNA binding activity. J. Biol. Chem. 2000;275:10247–10255. doi: 10.1074/jbc.275.14.10247. [DOI] [PubMed] [Google Scholar]
- 100.González-García A., Mérida I., Martinez A.C., Carrera A.C. Intermediate affinity interleukin-2 receptor mediates survival via a phosphatidylinositol 3-kinase-dependent pathway. J. Biol. Chem. 1997;272:10220–10226. doi: 10.1074/jbc.272.15.10220. [DOI] [PubMed] [Google Scholar]
- 101.Hand T.W., Cui W., Jung Y.W., Sefik E., Joshi N.S., Chandele A., et al. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16601–16606. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.DiToro D., Winstead C.J., Pham D., Witte S., Andargachew R., Singer J.R., et al. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science. 2018;361 doi: 10.1126/science.aao2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liao W., Lin J.X., Leonard W.J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pepper M., Pagán A.J., Igyártó B.Z., Taylor J.J., Jenkins M.K. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Colamatteo A., Carbone F., Bruzzaniti S., Galgani M., Fusco C., Maniscalco G.T., et al. Molecular mechanisms controlling Foxp3 expression in Health and autoimmunity: from epigenetic to post-translational regulation. Front Immunol. 2019;10:3136. doi: 10.3389/fimmu.2019.03136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fontenot J.D., Rasmussen J.P., Gavin M.A., Rudensky A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 107.Fan M.Y., Low J.S., Tanimine N., Finn K.K., Priyadharshini B., Germana S.K., et al. Differential roles of IL-2 signaling in developing versus mature tregs. Cell Rep. 2018;25:1204–1213.e4. doi: 10.1016/j.celrep.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Owen D.L., Sjaastad L.E., Farrar M.A. Regulatory T cell development in the thymus. J. Immunol. 2019;203:2031–2041. doi: 10.4049/jimmunol.1900662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lio C.W., Hsieh C.S. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zou T., Satake A., Corbo-Rodgers E., Schmidt A.M., Farrar M.A., Maltzman J.S., et al. Cutting edge: IL-2 signals determine the degree of TCR signaling necessary to support regulatory T cell proliferation in vivo. J. Immunol. 2012;189:28–32. doi: 10.4049/jimmunol.1200507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J., Zhao X., Wan Y.Y. Intricacies of TGF-β signaling in Treg and Th17 cell biology. Cell Mol Immunol. 2023;20:1002–1022. doi: 10.1038/s41423-023-01036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tischner D., Wiegers G.J., Fiegl H., Drach M., Villunger A. Mutual antagonism of TGF-beta and Interleukin-2 in cell survival and lineage commitment of induced regulatory T cells. Cell Death Differ. 2012;19:1277–1287. doi: 10.1038/cdd.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schmitt E.G., Williams C.B. Generation and function of induced regulatory T cells. Front. Immunol. 2013;4:152. doi: 10.3389/fimmu.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Freudenberg K., Lindner N., Dohnke S., Garbe A.I., Schallenberg S., Kretschmer K. Critical role of TGF-β and IL-2 receptor signaling in Foxp3 induction by an inhibitor of DNA methylation. Front. Immunol. 2018;9:125. doi: 10.3389/fimmu.2018.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Antov A., Yang L., Vig M., Baltimore D., Van Parijs L. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. J. Immunol. 2003;171:3435–3441. doi: 10.4049/jimmunol.171.7.3435. [DOI] [PubMed] [Google Scholar]
- 116.Burchill M.A., Yang J., Vogtenhuber C., Blazar B.R., Farrar M.A. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 117.Vogtenhuber C., Bucher C., Highfill S.L., Koch L.K., Goren E., Panoskaltsis-Mortari A., et al. Constitutively active Stat5b in CD4+ T cells inhibits graft-versus-host disease lethality associated with increased regulatory T-cell potency and decreased T effector cell responses. Blood. 2010;116:466–474. doi: 10.1182/blood-2009-11-252825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Elias S., Rudensky A.Y. Therapeutic use of regulatory T cells for graft-versus-host disease. Br. J. Haematol. 2019;187:25–38. doi: 10.1111/bjh.16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jenks J.A., Seki S., Kanai T., Huang J., Morgan A.A., Scalco R.C., et al. Differentiating the roles of STAT5B and STAT5A in human CD4+ T cells. Clin. Immunol. 2013;148:227–236. doi: 10.1016/j.clim.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fichna M., Zurawek M., Fichna P., Ziółkowska-Suchanek I., Januszkiewicz D., Nowak J. Polymorphic variant at the IL2 region is associated with type 1 diabetes and may affect serum levels of interleukin-2. Mol. Biol. Rep. 2013;40:6957–6963. doi: 10.1007/s11033-013-2815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hartmann F.J., Khademi M., Aram J., Ammann S., Kockum I., Constantinescu C., et al. Multiple sclerosis-associated IL2RA polymorphism controls GM-CSF production in human TH cells. Nat. Commun. 2014;5:5056. doi: 10.1038/ncomms6056. [DOI] [PubMed] [Google Scholar]
- 122.Keindl M., Davies R., Bergum B., Brun J.G., Hammenfors D., Jonsson R., et al. Impaired activation of STAT5 upon IL-2 stimulation in Tregs and elevated sIL-2R in Sjögren's syndrome. Arthritis Res. Ther. 2022;24:101. doi: 10.1186/s13075-022-02769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lowe C.E., Cooper J.D., Brusko T., Walker N.M., Smyth D.J., Bailey R., et al. Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat. Genet. 2007;39:1074–1082. doi: 10.1038/ng2102. [DOI] [PubMed] [Google Scholar]
- 124.Garg G., Tyler J.R., Yang J.H., Cutler A.J., Downes K., Pekalski M., et al. Type 1 diabetes-associated IL2RA variation lowers IL-2 signaling and contributes to diminished CD4+CD25+ regulatory T cell function. J. Immunol. 2012;188:4644–4653. doi: 10.4049/jimmunol.1100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gootjes C., Zwaginga J.J., Roep B.O., Nikolic T. Functional impact of risk gene variants on the autoimmune responses in type 1 diabetes. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.886736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang X.X., Chen T. Meta-analysis of the association of IL2RA polymorphisms rs2104286 and rs12722489 with multiple sclerosis risk. Immunol. Invest. 2018;47:431–442. doi: 10.1080/08820139.2018.1425699. [DOI] [PubMed] [Google Scholar]
- 127.Kabelitz D., Janssen O. Antigen-induced death of T-lymphocytes. Front Biosci. 1997;2:d61–d77. doi: 10.2741/a175. [DOI] [PubMed] [Google Scholar]
- 128.Cousens L.P., Orange J.S., Biron C.A. Endogenous IL-2 contributes to T cell expansion and IFN-gamma production during lymphocytic choriomeningitis virus infection. J. Immunol. 1995;155:5690–5699. [PubMed] [Google Scholar]
- 129.Refaeli Y., Van Parijs L., Alexander S.I., Abbas A.K. Interferon gamma is required for activation-induced death of T lymphocytes. J. Exp. Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Courtney A.H., Shvets A.A., Lu W., Griffante G., Mollenauer M., Horkova V., et al. CD45 functions as a signaling gatekeeper in T cells. Sci. Signal. 2019;12 doi: 10.1126/scisignal.aaw8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Castro-Sanchez P., Teagle A.R., Prade S., Zamoyska R. Modulation of TCR signaling by tyrosine phosphatases: from autoimmunity to immunotherapy. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.608747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Furlan G., Minowa T., Hanagata N., Kataoka-Hamai C., Kaizuka Y. Phosphatase CD45 both positively and negatively regulates T cell receptor phosphorylation in reconstituted membrane protein clusters. J. Biol. Chem. 2014;289:28514–28525. doi: 10.1074/jbc.M114.574319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kishihara K., Penninger J., Wallace V.A., Kündig T.M., Kawai K., Wakeham A., et al. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 134.Irie-Sasaki J., Sasaki T., Matsumoto W., Opavsky A., Cheng M., Welstead G., et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001;409:349–354. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- 135.Holmes N. CD45: all is not yet crystal clear. Immunology. 2006;117:145–155. doi: 10.1111/j.1365-2567.2005.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Volkov D.V., Stepanova V.M., Rubtsov Y.P., Stepanov A.V., Gabibov A.G. Protein tyrosine phosphatase CD45 as an immunity regulator and a potential effector of CAR-T therapy. Acta Naturae. 2023;15:17–26. doi: 10.32607/actanaturae.25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zikherman J., Weiss A. Alternative splicing of CD45: the tip of the iceberg. Immunity. 2008;29:839–841. doi: 10.1016/j.immuni.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 138.Altin J.G., Sloan E.K. The role of CD45 and CD45-associated molecules in T cell activation. Immunol. Cell Biol. 1997;75:430–445. doi: 10.1038/icb.1997.68. [DOI] [PubMed] [Google Scholar]
- 139.Hermiston M.L., Xu Z., Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 140.Basadonna G.P., Auersvald L., Khuong C.Q., Zheng X.X., Kashio N., Zekzer D., et al. Antibody-mediated targeting of CD45 isoforms: a novel immunotherapeutic strategy. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3821–3826. doi: 10.1073/pnas.95.7.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fecteau S., Basadonna G.P., Freitas A., Ariyan C., Sayegh M.H., Rothstein D.M. CTLA-4 up-regulation plays a role in tolerance mediated by CD45. Nat. Immunol. 2001;2:58–63. doi: 10.1038/83175. [DOI] [PubMed] [Google Scholar]
- 142.Camirand G., Wang Y., Lu Y., Wan Y.Y., Lin Y., Deng S., et al. CD45 ligation expands Tregs by promoting interactions with DCs. J. Clin. Invest. 2014;124:4603–4613. doi: 10.1172/JCI74087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van Vliet S.J., Gringhuis S.I., Geijtenbeek T.B., van Kooyk Y. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat. Immunol. 2006;7:1200–1208. doi: 10.1038/ni1390. [DOI] [PubMed] [Google Scholar]
- 144.Jacobsen M., Schweer D., Ziegler A., Gaber R., Schock S., Schwinzer R., et al. A point mutation in PTPRC is associated with the development of multiple sclerosis. Nat. Genet. 2000;26:495–499. doi: 10.1038/82659. [DOI] [PubMed] [Google Scholar]
- 145.Rheinländer A., Schraven B., Bommhardt U. CD45 in human physiology and clinical medicine. Immunol. Lett. 2018;196:22–32. doi: 10.1016/j.imlet.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 146.Vogel A., Strassburg C.P., Manns M.P. 77 C/G mutation in the tyrosine phosphatase CD45 gene and autoimmune hepatitis: evidence for a genetic link. Genes Immun. 2003;4:79–81. doi: 10.1038/sj.gene.6363918. [DOI] [PubMed] [Google Scholar]
- 147.Neidhart M., Pataki F., Michel B.A., Fehr K. CD45 isoforms expression on CD4+ and CD8+ peripheral blood T-lymphocytes is related to auto-immune processes and hematological manifestations in systemic lupus erythematosus. Schweiz. Med. Wochenschr. 1996;126:1922–1925. [PubMed] [Google Scholar]
- 148.Socha P., Michałkiewicz J., Stachowski J., Pawłowska J., Jankowska I., Barth C., et al. Deficiency of the expression of CD45RA isoform of CD45 common leukocyte antigen in CD4+ T lymphocytes in children with infantile cholestasis. Immunol. Lett. 2001;75:179–184. doi: 10.1016/s0165-2478(00)00305-9. [DOI] [PubMed] [Google Scholar]
- 149.Dong Z., Zhang B., Rong J., Yang X., Wang Y., Zhang Q., et al. The aberrant expression of CD45 isoforms and levels of sex hormones in systemic lupus erythematosus. Clin. Rheumatol. 2022;41:1087–1093. doi: 10.1007/s10067-021-05934-x. [DOI] [PubMed] [Google Scholar]