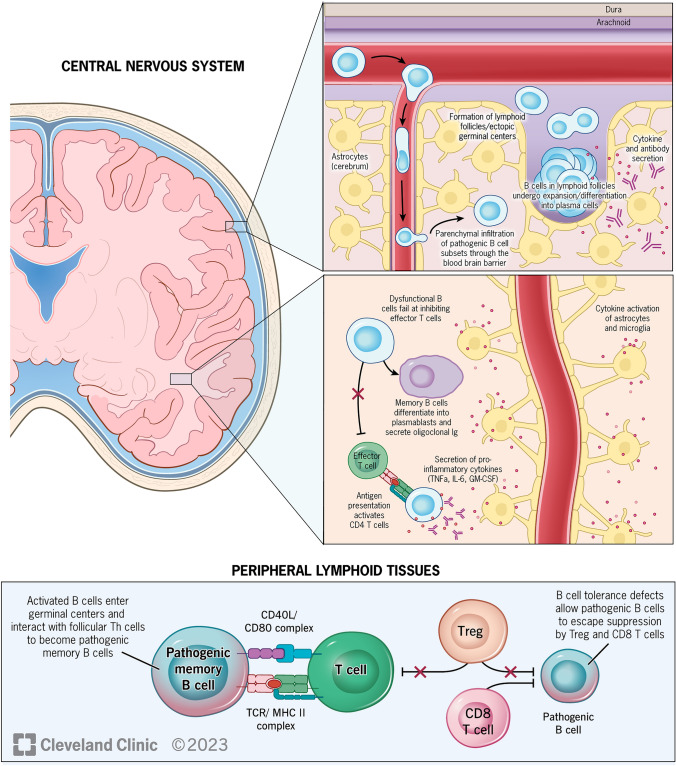
Fig. 1.
B-cell targets in CNS and periphery [4]. B-cell populations in the periphery, which contain tolerance defects, escape suppression by Treg and CD8+ T cells and enter germinal centers, where they differentiate into pathogenic memory B cells through interactions with follicular Th cells. Subsets of these pathogenic memory B cells, which express chemokine receptors CXCR3 and CCR6, proinflammatory cytokines, and adhesion molecule VLA-4, then infiltrate CNS through the blood-brain barrier, where they encounter T cells in follicle-like structures, leading to clonal expansion. Once inside the CNS, memory B cells may become plasmablasts, which secrete antibodies. Pathogenic B cells also secrete pro-inflammatory cytokines, which leads to activation of astrocytes and microglia, failure of effector T-cell inhibition, and activation of CD4+ T cells. CD cluster of differentiation, CNS central nervous system, GM-CSF granulocyte-macrophage colony-stimulating factor, IL interleukin, Th T helper, TNF tumor necrosis factor, Treg T regulatory