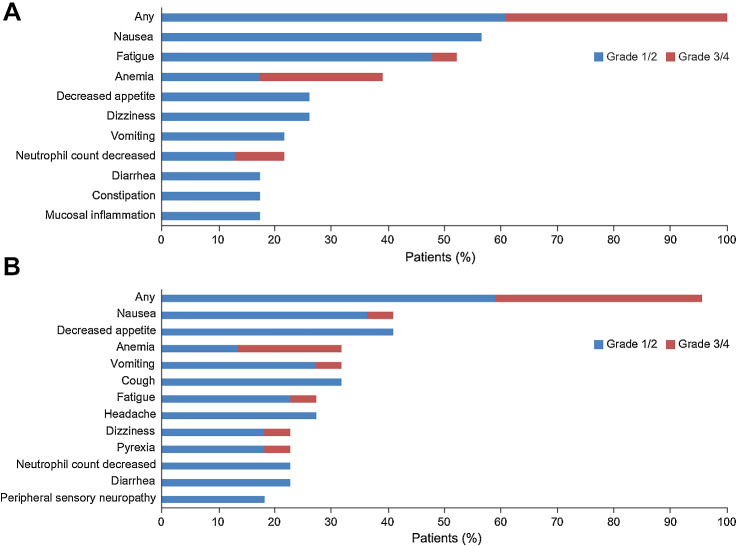
Figure 4.
Most common adverse events (AE; grade ≥3 in any patient or any grade in ≥15% of patients). A, Olaparib alone. B, Olaparib plus durvalumab. Additional grade 3/4 AEs in the olaparib-alone arm comprised lymphocyte count decreased, cough, hypophosphatemia, and neutropenia, each in 1 patient (4%). Additional grade 3/4 AEs in the olaparib plus durvalumab combination arm comprised non-cardiac chest pain, neutropenia, pneumonia, upper abdominal pain, amylase increased, white blood cell count decreased, and lipase increased, each in only 1 patient (5%).