Abstract
Purpose
Severe eosinophilic asthma (SEA) patients often present overlapping inflammatory features rendering them eligible for multiple biologic therapies; switching biologic treatment is a strategy adopted to optimize asthma control when patients show partial or no response to previous biologics.
Patients and Methods
ANANKE is a retrospective, multicenter Italian study (NCT04272463). Here, we outline the characteristics and long-term clinical outcomes in naïve-to-biologics and biologics-experienced patients treated with benralizumab for up to 96 weeks. Bio-experienced patients were split into omalizumab and mepolizumab subsets according to the type of biologic previously used.
Results
A total of 124 (76.5%) naïve and 38 (23.5%) bio-experienced patients were evaluated at index date; 13 patients (34.2%) switched from mepolizumab, 21 patients (55.3%) switched from omalizumab, and four patients (10.5%) received both biologics. The mepolizumab subset was characterized by the longest SEA duration (median of 4.6 years), the highest prevalence of chronic rhinosinusitis with nasal polyposis (CRSwNP) (76.5%), and the greatest oral corticosteroid (OCS) daily dosage (median of 25 mg prednisone equivalent). The omalizumab group showed the highest severe annual exacerbation rate (AER) (1.70). At 96 weeks, treatment with benralizumab reduced any and severe AER by more than 87% and 94%, respectively, across all groups. Lung function was overall preserved, with major improvements observed in the mepolizumab group, which also revealed a 100% drop of the median OCS dose. Asthma Control Test (ACT) score improved in the naïve group while its increment was more variable in bio-experienced patients; among these, a marked difference was noticed between omalizumab and mepolizumab subsets (median ACT score of 23.5 and 18, respectively).
Conclusion
Benralizumab promotes durable and profound clinical benefits in naïve and bio-experienced groups, indicating that a nearly complete depletion of eosinophils is highly beneficial in the control of SEA, independently of previous biologic use.
Keywords: benralizumab, asthma, eosinophils, switch, long-term
Background
Severe eosinophilic asthma (SEA) is a complex chronic disease of the lungs in which the inflammatory process is driven predominantly by eosinophils. Severe exacerbations and inadequate lung function contribute to the poor quality of life that SEA patients experience.1–3 In addition to the typical increase in blood and sputum eosinophil counts,4 mainly sustained by interleukin (IL)5,5,6 SEA patients can also present other clinical features, such as atopy and increased levels of multiple inflammatory biomarkers (eg, fractional exhaled nitric oxide [FeNO], IL4, IL13), which are governed by distinct but overlapping T2 inflammatory pathways.7 Oral corticosteroids (OCS) suppress the ongoing T2 inflammation in a non-specific manner, hence are highly efficacious in reducing asthma symptoms. Based on the several adverse events that OCS can cause, their use is strongly condemned by international guidelines, which endorse their use as a “last resort” only.8 In the last decades, the advance of several biologic drugs targeting selected T2 inflammatory players encouraged the minimization of OCS treatment and emphasized the use of a personalized approach, in an effort to distinguish not only the SA phenotype but also the underlying individual endotype.
To date, there are six monoclonal antibodies (mAbs) authorized for the treatment of severe asthma (SA): while omalizumab is intended to treat allergic SA patients,9 mepolizumab, reslizumab, and benralizumab are indicated for SEA,10–12 dupilumab is approved for T2 SA,13 and tezepelumab use has been recently authorized in a broader SA population.14 Patients showing overlapping T2 features can be eligible for multiple biologics; choosing the right treatment for these patients is difficult as no head-to-head randomized controlled trials have been conducted to compare the different mAbs in terms of efficacy and safety. Only a few real-life studies have investigated the effects of mepolizumab and benralizumab, reporting either no clinically relevant differences15 or a higher number of patients treated with benralizumab in terms of disease clinical remission.16 Although most patients largely benefit from the prescribed biologic agent, the extent of the response can vary.17 Therefore, a thorough characterization of the patient searching for specific clinical characteristics, comorbidities, and/or biomarkers that could predict the most effective biologic is recommended.8,18 Chronic rhinosinusitis with nasal polyps (CRSwNP) has been linked to an enhanced response to benralizumab in reducing annual exacerbation rate (AER)19,20 and improving lung function;21,22 dupilumab represents the first choice in patients with high FeNO23 but should be avoided if blood eosinophil count (BEC) is higher than 1,500 cells/mm;3,8 the presence of atopy could favor the choice of omalizumab, although it does not preclude beneficial effects from other biologics in patients with overlapping T2 features.24–28
Despite the efforts to select the most effective biologic to treat SA, the rate of patients undergoing a biologic switch because of poorly controlled asthma is significant. A recent real-world analysis examining the patterns of biologics use and switch among 3,531 patients showed that the initial biologic was maintained in most cases (79% of patients), while it was either stopped or switched in 10.2% and 10.8% of patients, respectively.29 Higher percentages of patients who switched biologic have been observed in smaller studies (up to 43.3%).30,31 These results underline the importance of routinely examining the patient and re-assess their response to the biologic treatment. As recommended by Global Strategy for Asthma Management (GINA) guidelines, the therapy should be stopped if patients do not respond and a biologic switch should be considered by the practitioner.8
Among all available biologics, benralizumab is the only mAb that ensures an extensive eosinophil apoptosis through enhanced antibody-dependent cell cytotoxicity (ADCC), activated via the simultaneous binding of IL5 receptor alpha (IL5Rα) and FcγIIIRa receptor, predominantly expressed by eosinophils and natural killer (NK) cells, respectively.32 A recent study described a series of immunological modifications taking place in SEA patients treated with benralizumab, including increased NK cell proliferation, maturation and cytotoxic activity, and modulation of T cell subsets. These findings further elucidated the benralizumab unique mode of action, proving its ability to restore immune functions to levels comparable to those seen in untreated healthy controls.33 ANANKE (NCT04272463)34,35 is a large retrospective study evaluating the characteristics of Italian SEA patients treated with benralizumab and their clinical response. A number of post hoc analyses have been conducted so far; among these, Caruso et al examined the differences between patients who received benralizumab as the first-choice biologic (naïve patients) and patients switching to benralizumab after they received other biologics (bio-experienced patients).36 The results revealed that benralizumab was highly efficacious in both naïve and bio-experienced groups; clinical outcomes were comparable even though bio-experienced patients were characterized by higher severe AER and a protracted duration of SEA compared to naïve patients. More recently, we confirmed the durable effectiveness of benralizumab in reducing any and severe AER by more than 90% in both naïve and bio-experienced patients, up to 96 weeks of treatment.37
In light of what has been previously reported, we conducted a post hoc analysis to further describe the outcomes of long-term response to benralizumab in patients with severe eosinophilic asthma, irrespective from previous biologics treatment (naïve and bio-experienced). Moreover, bio-experienced patients were split into two subsets according to the biologic(s) previously received (omalizumab and/or mepolizumab); these were added to the comparative analysis to evaluate if and how the biologic(s) previously used influenced the response to benralizumab.
Materials and Methods
Study Design
ANANKE (NCT04272463) is an Italian multi-center, observational, retrospective study.34 The centers participating in the ANANKE study are listed in Supplementary File 1. Briefly, benralizumab was administered to SEA patients in accordance with clinical practice or as part of the Italian Sampling Programme; their enrolment occurred at least 3 months after the index date (when benralizumab treatment was started). Differently from the initial design, the observation period of the study was extended up to 96 weeks; as a consequence, patients agreed to participate in the extended study period and signed the related privacy and informed consent forms. After initiation of benralizumab treatment, data were collected at weeks 4, 16, 24, 48, and 96. ANANKE was performed in agreement with the ethical principles outlined in the Declaration of Helsinki as well as with the regulations and policies governing the clinical practice in Italy. Ethics committees/institutional review boards of all sites involved in the study approved the study.
Patient Population
Inclusion and exclusion criteria have been previously detailed.35 Briefly, enrolled patients were adults (≥18 years old), diagnosed with SEA and requiring treatment with high doses of background medications (inhaled corticosteroids [ICS] and a long-acting b2-agonist [LABA]), with or without additional asthma controllers. Participants were required to have initiated Benralizumab therapy at least 3 months prior to enrollment, with at least one benralizumab injection received, and their hospital medical charts had to be available from 12 months before the index date. Patients were excluded from the ANANKE study if they took part in other studies dictating a particular patient management method that differed from the site’s normal clinical practice after the enrollment visit.
Eligible patients satisfied all inclusion, exclusion, and amendment criteria. Evaluable patients were all eligible patients with BEC data available at index date.
Outcomes
Data were gathered from hospital medical records and entered into the electronic case report form (eCRF) as per clinical practice.
The primary endpoint was the description of patients’ characteristics at index date (ie, when benralizumab treatment was started). Specifically, the data at index date were gathered throughout the 12 months before the start of benralizumab treatment and consisted of age, gender, body mass index (BMI), tobacco usage, age at asthma diagnosis, duration of asthma and duration of SEA, diagnosis of atopy (in presence of a positive skin prick test (SPT) for an allergen, either perennial and/or seasonal), diagnosis of concomitant illnesses (comorbidities associated with either asthma or OCS use, and any other conditions deemed meaningful by the treating clinician), BEC, and blood level of serum immunoglobulin E (IgE), respiratory parameters, control of asthma symptoms (measured with the Asthma Control Test [ACT]), use of asthma treatments (background and chronic therapies, including OCS, and any biologics used during the 12 months prior to benralizumab initiation), any and severe AER. Any AER accounted for all the clinically significant asthma exacerbations determined by a physician, while severe AER included all exacerbations that further deteriorated asthma symptoms, requiring either: a) systemic corticosteroids, administered for at least 3 days, or a transient increment in the dosage of maintenance OCS; b) a visit to the emergency department (shorter than 24 hours) during which systemic corticosteroids were administered; or c) hospitalization (longer than 24 hours).
In addition, a range of secondary endpoints was evaluated during benralizumab treatment (ie, between the index date and 96 weeks), and included outcomes recorded at multiple time points (4, 16, 24, and 48 weeks) when available. Changes in the following clinical and laboratory outcomes were evaluated over time:
BEC (to confirm benralizumab performance);
AER (any and severe), proportion of patients eliminating any and severe exacerbations;
ACT score, the proportion of patients reaching ACT score ≥20 (well-controlled asthma);
Respiratory parameters (forced expiratory volume in the first second [FEV1], forced vital capacity [FVC], measured both pre- and post-bronchodilator [BD]);
Treatment with OCS and their dosage (expressed as prednisone-equivalent mg per day), proportion of patients reducing or permanently interrupting OCS; and
Discontinuation to benralizumab treatment.
Both primary and secondary outcomes were analyzed in naïve patients (not treated with any biologic for asthma before index date) and bio-experienced patients (previously treated with one or more biologics for asthma before the index date). Within the bio-experienced patients, two subsets were considered for analysis based on the type of biologic received before benralizumab: the first group included patients who previously received omalizumab only (omalizumab group), the second group consisted of patients who were previously treated with mepolizumab, including patients who firstly received omalizumab and then switched to mepolizumab before receiving benralizumab. These groups were included in the post hoc analyses and compared with naïve and bio-experienced patients.
Statistical Analysis
Statistical analyses were extensively described by Menzella et al.35 Briefly, descriptive analyses were performed; no formal hypothesis was explicitly determined due to the study’s observational nature. Data are expressed as mean ± standard deviation (SD) or absolute numbers and frequencies. Median (IQR) was used instead of mean ± SD if data distribution was highly variable.
Demographic and clinical characteristics at the index date were assessed in evaluable patients (naïve and bio-experienced patients, N=124 and N=38, respectively); secondary endpoints were assessed in evaluable patients for secondary analyses at 48 (naïve and bio-experienced patients, N=110 and N=35, respectively) and 96 weeks (naïve and bio-experienced patients, N=86 and N=27, respectively).
SAS for Windows Version 9.4 and SAS Enterprise Guide 7.12 (SAS Institute, Cary, NC, USA) were used to conduct statistical analyses.
Results
Patient Distribution
As previously described, 218 patients diagnosed with SEA were enrolled in the ANANKE study.35 Based the inclusion and exclusion criteria, 167 (76.6%) patients were eligible for evaluation during the extended period. Reasons for non-eligibility were: benralizumab initiation did not take place at least 3 months before enrollment; absence of signed informed consent and privacy forms (including amendments related to the extended observation period); patients did not receive ICS and LABA before starting benralizumab treatment.
Because five patients were missing BEC values, the number of evaluable patients at index date was 162; of these, 124 (76.5%) were naïve and 38 (23.5%) were already treated with biologics before switching to benralizumab (bio-experienced). Among the latter group, 13 patients (34.2%) received mepolizumab, 21 patients (55.3%) received omalizumab, and four patients (10.5%) received omalizumab as first treatment, which was followed by mepolizumab. Because of the low number of patients undergoing double switching (from omalizumab to mepolizumab, and then from mepolizumab to benralizumab, N=4), these patients were combined with the 13 patients who received mepolizumab only and formed the mepolizumab group (N=17, representing 44.7% of all bio-experienced patients).
Among evaluable patients at index date, 145 participants were judged evaluable for secondary analyses at 48 weeks. Among these, there were 110 (67.9%) naïve patients and 35 (21.6%) bio-experienced patients (with 20 [57.1%] and 15 [42.9%] patients in the omalizumab and mepolizumab groups, respectively). At 96 weeks, 113 patients were judged evaluable for secondary analysis, with 86 (53.0%) naïve patients and 27 (16.7%) bio-experienced patients (with 17 [62.9%] and 10 [37.0%] patients in the omalizumab and mepolizumab groups, respectively).
Characteristics of Patients at Index Date According to Previous Biologics Use
The ANANKE patient population eligible for extended analysis presented characteristics compatible with the late onset, eosinophilic-driven SA phenotype.37 The key features of naïve and bio-experienced patients at index date (ie, collected during the 12 months before benralizumab initiation) have already been reported; in general, asthma clinical control appeared worse in bio-experienced patients in terms of severe AER, OCS dosage, and OCS-related comorbidities (Table 1).37 The characteristics of bio-experienced patients divided into omalizumab and mepolizumab subsets are detailed below.
Table 1.
Socio-Demographic and Clinical Characteristics of Naïve and Bio-Experienced Patients, Including Omalizumab and Mepolizumab Subsets, Collected Before Initiating Benralizumab Treatment
| Characteristics at Index Date | Naïve (N=124) | Bio-Experienced (N=38) | Omalizumab (N=21) | Mepolizumab (N=17) (Including Four Patients Switched from Omalizumab) |
|---|---|---|---|---|
| Age (years) | 56.6 ± 12.3 | 54.3 ± 13.7 | 59.1 ± 10.5 | 48.3 ± 15.0 |
| Gender (female) | 82 (66.1) | 17 (44.7) | 8 (38.1) | 9 (52.9) |
| BMI classes | ||||
| Underweight/Normal | 47 (37.9) | 11 (28.9) | 4 (19.0) | 7 (41.2) |
| Overweight | 46 (37.1) | 16 (42.1) | 11 (52.4) | 5 (29.4) |
| Obese | 19 (15.3) | 7 (18.4) | 5 (23.8) | 2 (11.8) |
| Unknown | 12 (9.7) | 4 (10.5) | 1 (4.8) | 3 (17.6) |
| Smoking status | ||||
| Current smokers | 4 (3.2) | 1 (2.6) | 0 (0.0) | 1 (5.9) |
| Past smokers | 31 (25.0) | 13 (34.2) | 8 (38.1) | 5 (29.4) |
| Unknown | 4 (3.2) | 3 (7.9) | 1 (4.8) | 2 (11.8) |
| Asthma duration (years) (N=123, 38, 21, 17) | 13.5 (8.1–26.4) | 14.0 (8.5–20.5) | 20.3 (9.4–30.1) | 10.2 (6.5–14.8) |
| SEA duration (years) (N=120, 38, 21, 17) | 1.6 (1.0–3.0) | 3.5 (1.6–6.5) | 2.1 (1.1–4.5) | 4.6 (2.5–9.2) |
| Patients positive to ≥ 1 allergen | 56 (45.2) | 21 (55.3) | 12 (57.1) | 9 (52.9) |
| BEC (cells/mm3) | 605 (440–915) | 550 (300–756) | 550 (400–770) | 550 (230–700) |
| Total serum IgE (IU/mL) (N=69, 22, 13, 9) | 161 (73–474) | 308 (128–620) | 326 (78–520) | 215 (172–774) |
| Duration of previous biologic treatment (months) | N/A | 21.0 (10.6–52.9) | 35.3 (15.9–92.0) | 15.4 (10.6–27.4) |
| Any comorbidities | 107 (86.3) | 34 (89.5) | 17 (81.0) | 17 (100.0) |
| ≥1 any asthma-related | 71 (57.3) | 20 (52.6) | 9 (42.9) | 11 (64.7) |
| CRSwNP (current or past) | 68 (54.8) | 18 (47.4) | 5 (23.8) | 13 (76.5) |
| CRSsNP | 32 (25.8) | 11 (28.9) | 6 (28.6) | 5 (29.4) |
| ≥1 current OCS-related | 44 (35.5) | 20 (52.6) | 11 (52.4) | 9 (52.9) |
| ≥1 other ongoing | 20 (16.1) | 7 (18.4) | 4 (19.0) | 3 (17.6) |
| OCS users (for asthma treatment) | 30 (24.2) | 11 (28.9) | 4 (19.0) | 7 (41.2) |
| OCS daily dose (mg) (N=28, 11, 4, 7) | 8.1 (5.0–21.3) | 10.0 (5.0–25.0) | 5.0 (5.0–7.5) | 25.0 (10.0–25.0) |
| Exacerbations | ||||
| Patients with ≥1, any (N=117, 37, 20, 17) | 109 (93.2) | 35 (94.6) | 20 (100.0) | 15 (88.2) |
| Patients with ≥1, severe (N=117, 37, 20, 17) | 39 (33.3) | 18 (48.6) | 11 (55.0) | 7 (41.2) |
| AER, any (N=117, 37, 20, 17) | 4.15 | 3.95 | 3.80 | 4.12 |
| AER, severe (N=117, 37, 20, 17) | 0.81 | 1.51 | 1.70 | 1.29 |
| Lung function | ||||
| Pre-BD FEV1 (L) (N=82, 29, 18, 11) | 1.8 (1.4–2.5) | 2.1 (1.4–2.4) | 2.0 (1.4–2.4) | 2.2 (1.0–2.7) |
| Pre-BD FEV1 predicted (%) (N=83, 30, 18, 12) | 72.0 (54.0–85.0) | 71.0 (48.0–84.0) | 71.0 (48.0–81.0) | 71.5 (39.5–89.0) |
| Post-BD FEV1 (L) (N=58, 14, 7, 7) | 2.0 (1.4–2.7) | 2.1 (1.4–3.0) | 2.8 (1.3–3.4) | 1.9 (1.4–3.0) |
| Post-BD FEV1 predicted (%) (N=56, 14, 7, 7) | 73.0 (60.0–91.5) | 66.5 (47.0–110) | 90.0 (50.0–117) | 49.0 (42.0–97.0) |
| Pre-BD FVC (L) (N=80, 28, 18, 10) | 2.7 (2.2–3.4) | 3.0 (2.5–3.7) | 3.2 (2.7–3.9) | 2.9 (2.4–3.3) |
| Post-BD FVC (L) (N=56, 12, 7, 5) | 3.0 (2.3–3.8) | 3.3 (2.4–4.4) | 3.7 (2.4–5.0) | 2.9 (2.3–3.2) |
| ACT score (N=90, 30, 16, 14) | 14.0 (12.0–18.0) | 14.0 (12.0–17.0) | 15.0 (12.5–17.0) | 12.5 (10.0–15.0) |
Notes: Data were collected at index date and are expressed as N (%), mean ± SD, or median (IQR). Unless otherwise specified, N=124 patients were evaluated in the naïve group, N=38 patients in the bio-experienced group, N=21 patients in the omalizumab group, and N=17 patients in the mepolizumab group.
Abbreviations: N, number of patients; SEA, severe eosinophilic asthma; BEC, blood eosinophil count; IgE, immunoglobulin E; CRSwNP, chronic rhinosinusitis with nasal polyposis; CRSsNP, chronic rhinosinusitis without nasal polyps; OCS, oral corticosteroids; AER, annual exacerbation rate; Pre-BD FEV1, pre-bronchodilator forced expiratory volume in 1 second; Pre-BD FVC, pre-bronchodilator forced vital capacity; Post-BD FEV1, post-bronchodilator forced expiratory volume in 1 second; Post-BD FVC, post-bronchodilator forced vital capacity; ACT, asthma control test.
Patients were treated with omalizumab for 35.3 months (15.9–92.0) before receiving benralizumab, while patients in the mepolizumab group (including four patients who were treated with omalizumab prior to mepolizumab) received the anti IL5 mAb for a total period of 15.4 months (10.6–27.4). Overall, there was a median gap of 2.3 months (1.3–4.9) between the end of the previous biologic treatment and the start of benralizumab. In general, the mepolizumab group was characterized by a lower age compared with all the other groups (48.3 years ± 15.0); patients in this subset also showed a greater proportion of females and a lower proportion of overweight patients compared with the omalizumab group (females: 52.9% versus 38.1%; overweight: 29.4% versus 52.4%). Although asthma duration was shorter in the mepolizumab group (10.2 years, 6.5–14.8) compared with the other groups, this subset also featured the longest SEA duration (4.6 years, 2.5–9.2).
All mepolizumab-switched patients had at least one comorbidity, with CRSwNP being the most frequent asthma-related condition, affecting 13 out of 17 patients (76.5%). On the other hand, patients in the omalizumab group were predominantly positive for at least one allergen (57.1% of patients displayed allergen-specific IgE) and showed the highest median amount of total IgE levels (326 IU/mL, 78–520). As previously mentioned,36,37 OCS-related comorbidities were more prevalent in bio-experienced than naïve patients, with no difference between omalizumab and mepolizumab-treated subsets (52.4% and 52.9%, respectively), despite the mepolizumab group showing the highest proportion of OCS users (41.2%) and OCS daily dose (25.0 mg, 10.0–25.0).
An identical median BEC of 550 cells/mm3 was observed in both omalizumab and mepolizumab groups. Of note, BEC might have been measured before the start of mepolizumab treatment in some patients (characteristics at index date were measured throughout the 12 months before the start of benralizumab treatment and some patients were treated with mepolizumab only for a few months before switching to benralizumab). As expected, the omalizumab group displayed the highest level of total serum IgE (326.0 IU/mL).
During the year before the start of benralizumab treatment, all patients in the omalizumab group had one or more exacerbations of any severity and 55% of patients had at least one or more severe exacerbations (versus 88.2% and 41.2% of patients in the mepolizumab group). Any AER was slightly lower in the omalizumab compared with the mepolizumab group (3.80 vs 4.12); on the contrary omalizumab-switched patients featured the highest severe AER (1.70) among all groups considered.
Poor respiratory function and control of asthma were confirmed in all groups; the mepolizumab group displayed the lowest values of post-BD FEV1 (1.9, 1.4–3.0), indicating the highest degree of airway obstruction, and the lowest values of ACT scores (12.5, 10.0–15.0).
Benralizumab Reduced Exacerbations Irrespective of Previous Biologics Use
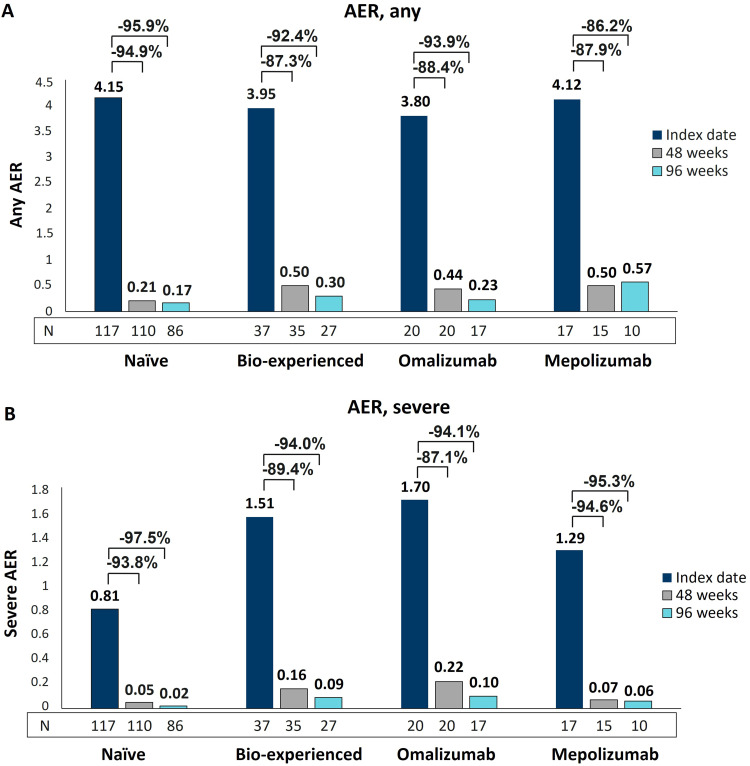
We have already shown the remarkable effectiveness of benralizumab in decreasing exacerbations in naïve and bio-experienced groups.36,37 Here, additional data are reported to describe the reduction in any and severe AER in omalizumab and mepolizumab groups after 96 weeks of treatment with benralizumab. As shown in Figure 1A, similarly to naïve and bio-experienced groups, the two switched groups had a consistent and sustained decrease in any AER, with reductions ranging from 86.2% (mepolizumab group) to 93.9% (omalizumab group) at 96 weeks. The most profound effects were related to severe AER, which declined in all groups in a progressive manner and at a nearly identical extent (from 94.1% in the omalizumab group to 97.5% in naïve patients at 96 weeks) (Figure 1B). As shown in Table 2, the percentage of patients free from severe exacerbations at 48 weeks increased in all groups, and the results were maintained at 96 weeks. In more detail, 66.7% of naïve patients were already free from severe exacerbations at the index date and almost all of them (97.7%) did not experience severe exacerbations for up to 96 weeks (+31% patients free from severe exacerbations). Similar increases were observed in the bio-experienced group and the subset of mepolizumab-treated patients (+33.8% and +31.2% patients respectively, at 96 weeks). The omalizumab group was characterized by the lowest proportion of patients without severe exacerbations at index date (45%), nevertheless, their percentage increased up to 82.4% at 96 weeks (+37.4% patients).
Figure 1.
AER reduction during benralizumab treatment in naïve, bio-experienced, omalizumab, and mepolizumab groups. Any (A) and severe (B) AER are shown at index date and after 48 and 96 weeks of treatment with benralizumab.
Table 2.
Proportion of Patients Free from Severe Exacerbations Before and During Benralizumab Treatment in Naïve, Bio-Experienced, Omalizumab, and Mepolizumab Groups
| Naïve (N=117, 110, 86) | Bio-Experienced (N=37, 35, 27) | Omalizumab (N=20, 20, 17) | Mepolizumab (N=17, 15, 10) | |
|---|---|---|---|---|
| Patients without severe exacerbations at index date, N (%) | 78 (66.7) | 19 (51.4) | 9 (45.0) | 10 (58.8) |
| Patients without severe exacerbations at 48 weeks, N (%) | 106 (96.4) | 30 (85.7) | 16 (80.0) | 14 (93.3) |
| Patients without severe exacerbations at 96 weeks, N (%) | 84 (97.7) | 23 (85.2) | 14 (82.4) | 9 (90.0) |
Notes: Data were collected at index date and at 48 and 96 weeks of benralizumab treatment; data are expressed as N (%).
Abbreviation: N, number of patients.
In line with the benralizumab mechanism of action (MoA), we confirmed the nearly complete depletion of blood eosinophils, which took place independently of previous biologics use (all groups showed a median BEC of 0 cells/mm3, maintained up to 96 weeks, data not shown).
Benralizumab Preserved Lung Function in Both Naïve and Bio-Experienced Groups with Pronounced Outcomes in the Mepolizumab Group
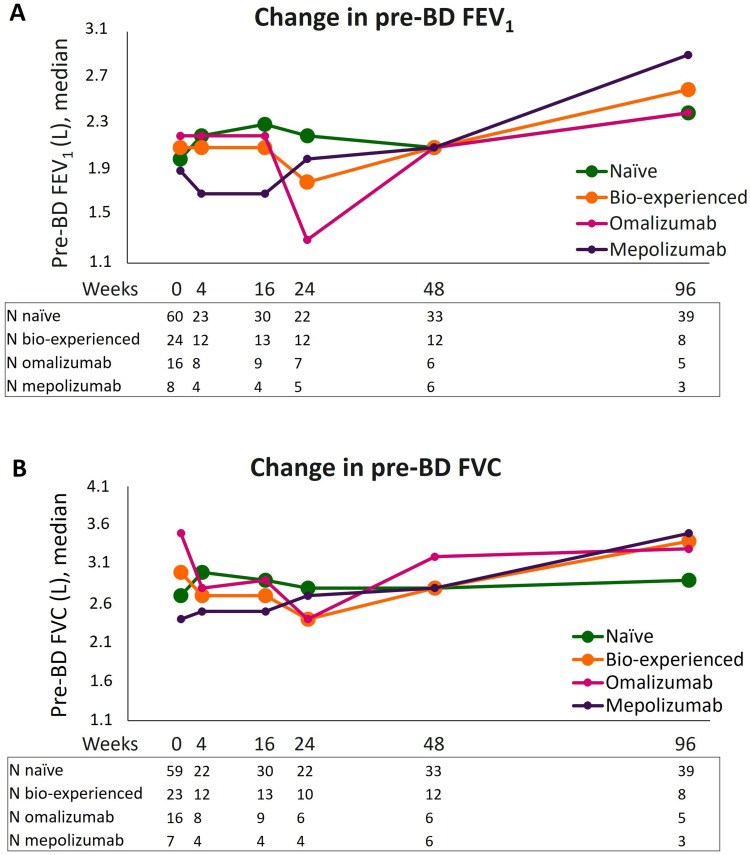
Changes in pre-BD FEV1 and FVC induced by benralizumab in naive and bio-experienced groups, including the subsets of omalizumab and mepolizumab-treated patients, are shown in Figure 2; median values related to the graphs are reported separately in Supplementary Table 1A and 1B. Both naïve and bio-experienced groups had net and comparable increments in pre-BD parameters measured from the start of benralizumab treatment to 96 weeks (median increases in pre-BD FEV1: +0.4 L and +0.5 L; median increases in pre-BD FVC: +0.2 L and +0.4 L, in naïve and bio-experienced patients, respectively). Although pre-BD FEV1 was ameliorated in both omalizumab and mepolizumab groups at 96 weeks, a more pronounced and steadier increase in both FEV1 and FVC was noticed in patients previously treated with mepolizumab. As a matter of fact, among all groups evaluated, this subset showed the greatest improvements at 96 weeks (median increase in pre-BD FEV1: +1.0 L and median increase in pre-BD FVC: +1.1 L). On the contrary, the worse response was observed in omalizumab-switched patients: both pre-BD measures profoundly decreased at 24 weeks compared with values at the index date; at 96 weeks, FEV1 improved by +0.2 L and a small decrease in FVC was registered (−0.2 L).
Figure 2.
Change in pre-BD respiratory parameters during benralizumab treatment in naïve, bio-experienced, omalizumab, and mepolizumab groups. Median pre-BD FEV1 (A) and pre-BD FVC (B) are shown at various time points (index date and 4, 16, 24, 48, and 96 weeks of treatment with benralizumab).
Post-BD FEV1 and FVC were generally preserved in all groups, with the most evident increments displayed once again by patients in the mepolizumab group at 96 weeks (Supplementary Figure 1, Supplementary Table 2A and 2B). Notably, the mepolizumab group displayed lower pre-BD FEV1 and FVC values (Figure 2A and B), as well as lower post-BD parameters (Table 1) compared with omalizumab-treated patients at index date, implying a more compromised respiratory function in this subset.
Benralizumab Ensured a Long-Term Reduction in OCS Use in Naïve and Bio-Experienced Groups with Prevalent Effects in the Mepolizumab Group
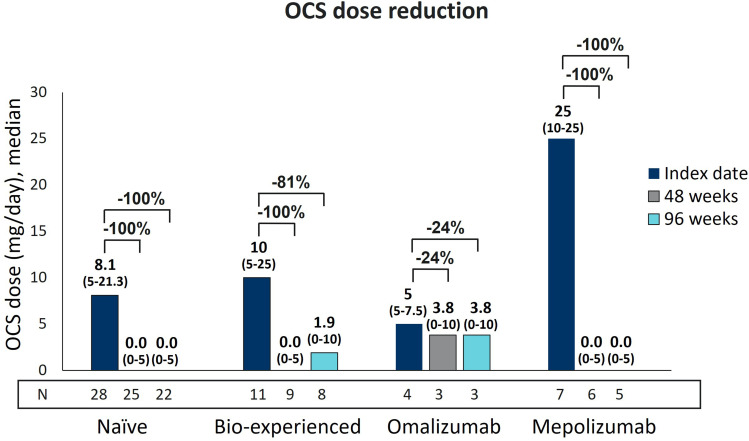
As shown in Figure 3, the median dose of OCS used by both naïve and bio-experienced groups was 0.0 mg/day (0–5) at 48 weeks. At 96 weeks, while the median OCS daily dose persisted at 0.0 mg in naïve patients, it slightly increased to 1.9 mg in bio-experienced patients. Such a result was not caused by an actual increase in the prescribed dose of OCS, but rather by missing data from one patient who stopped OCS at 48 weeks (the bio-experienced patients decreased from N=9 to N=8 at 96 weeks). In general, bio-experienced patients had a variable response to benralizumab treatment: when examining omalizumab and mepolizumab groups separately, clear differences were noticed between the two subsets. Patients previously treated with omalizumab had a modest decrease in median OCS dose (from 5 mg to 3.8 mg, 24% reduction measured at both 48 and 96 weeks, data from N=3), while a median OCS reduction of 100% was observed in the mepolizumab group (data from N=6 and N=5 at 48 and 96 weeks, respectively), despite the highest OCS starting dose (25 mg at index date).
Figure 3.
Long-term OCS reduction during benralizumab treatment in naïve, bio-experienced, omalizumab, and mepolizumab groups. OCS daily dose (mg of prednisone equivalent), expressed as median (IQR), is shown at index date and after 48 and 96 weeks of treatment with benralizumab.
Benralizumab successfully eliminated OCS use in 60% and 63.6% of naïve patients and 55.6% and 50% of patients in the bio-experienced group at 48 and 96 weeks (Table 3). Among bio-experienced patients, one out of three patients (33.3%) previously treated with omalizumab remained free from OCS use, whereas the number of patients interrupting OCS in the mepolizumab group were four (66.7%) and three (60%) at 48 and 96 weeks, respectively (96-week data are missing from one patient who interrupted OCS at 48 weeks).
Table 3.
Proportion of Patients Achieving OCS Dose Reduction and/or Interruption During Benralizumab Treatment in Naïve, Bio-Experienced, Omalizumab, and Mepolizumab Groups After 48 and 96 Weeks
| OCS Reduction from Index Date | Naive at 48 Weeks N (%) (N=25) | Naive at 96 Weeks N (%) (N=22) | Bio-Experienced at 48 Weeks N (%) (N=9) | Bio-Experienced at 96 Weeks N (%) (N=8) | Omalizumab at 48 Weeks N (%) (N=3) | Omalizumab at 96 Weeks N (%) (N=3) | Mepolizumab at 48 Weeks N (%) (N=6) | Mepolizumab at 96 Weeks N (%) (N=5) |
|---|---|---|---|---|---|---|---|---|
| Interruption | 13 (52.0) | 14 (63.6) | 5 (55.6) | 4 (50.0) | 1 (33.3) | 1 (33.3) | 4 (66.7) | 3 (60.0) |
| Any reduction (including interruption) | 15 (60.0) | 15 (68.2) | 6 (66.7) | 5 (62.5) | 2 (66.7) | 2 (66.7) | 4 (66.7) | 3 (60.0) |
| ≥90% dose reduction | 13 (52.0) | 14 (63.6) | 5 (55.6) | 4 (50.0) | 1 (33.3) | 1 (33.3) | 4 (66.7) | 3 (60.0) |
| ≥75% dose reduction | 13 (52.0) | 14 (63.6) | 5 (55.6) | 4 (50.0) | 1 (33.3) | 1 (33.3) | 4 (66.7) | 3 (60.0) |
| ≥25% dose reduction | 15 (60.0) | 15 (68.2) | 5 (55.6) | 4 (50.0) | 1 (33.3) | 1 (33.3) | 4 (66.7) | 3 (60.0) |
| No reduction | 10 (40.0) | 7 (31.8) | 3 (33.3) | 3 (37.5) | 1 (33.3) | 1 (33.3) | 2 (33.3) | 2 (40.0) |
Notes: OCS dosage is expressed as daily mg (prednisone equivalent). Data are expressed as N (%).
Abbreviations: N, number of patients; OCS, oral corticosteroids.
Benralizumab Induced a Superior Improvement in Asthma Control in Naïve and Omalizumab-Switched Patients
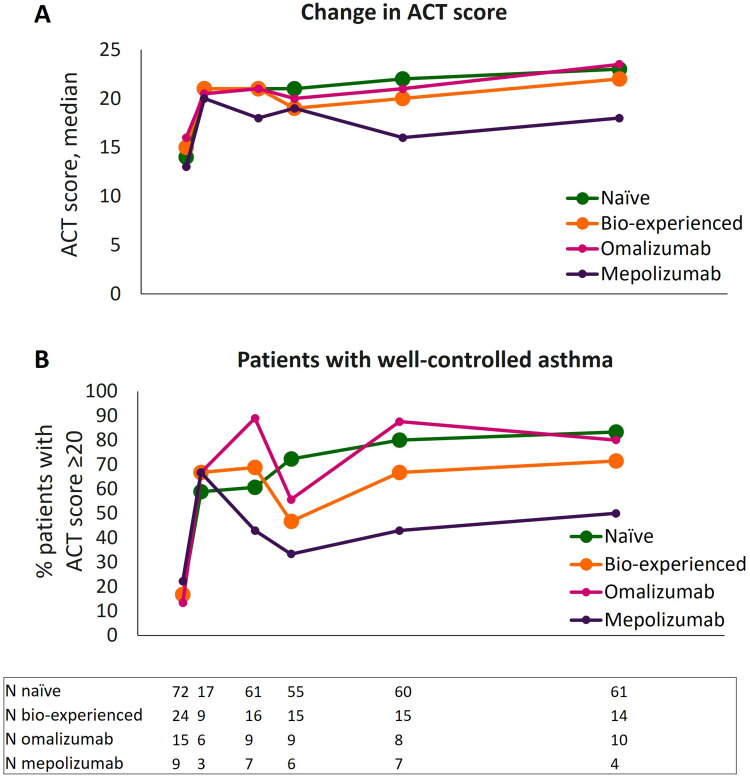
Change in asthma control was monitored via ACT during benralizumab treatment. As shown in Figure 4A and Supplementary Table 3A, ACT score quickly increased after only 4 weeks of treatment with benralizumab (ie, after a single injection), reaching median values equal to or greater than 20 in all groups. The rapid improvement in asthma control was maintained in naïve and bio-experienced groups, who obtained median ACT scores of 23 and 22, respectively, at 96 weeks. Within bio-experienced patients, the omalizumab group had a sharp increase in median ACT score that was maintained and reached a median score of 23.5 at 96 weeks. Although patients in the mepolizumab group also featured a rapid increment of asthma control (median ACT score=20 at 4 weeks), their ACT score was more variable and generally lower compared to the other groups.
Figure 4.
Improvement in asthma control during benralizumab treatment in naïve, bio-experienced, omalizumab, and mepolizumab groups. Median change in ACT score (A) and percentage of patients achieving a well-controlled asthma (ACT score ≥20) (B) are shown at various time points (index date and 4, 16, 24, 48, and 96 weeks of treatment with benralizumab).
The percentage of patients achieving an ACT score ≥20 (patients with well-controlled asthma, Figure 4B and Supplementary Table 3B) were also analyzed. Naïve and omalizumab groups had the highest percentages of patients with well-controlled asthma up to 96 weeks (80.3% and 80.0%, respectively), with a smaller percentage displayed by mepolizumab-treated patients (50%).
Discontinuation from Benralizumab Treatment
Of the eligible patients that were treated with benralizumab up to 96 weeks, six naïve (6.8%) and six bio-experienced patients (22.2%) (two and four patients in the omalizumab and mepolizumab groups, respectively) interrupted benralizumab. Efficacy failure was the primary reason leading to discontinuation across all groups (data not shown).
Discussion
A new post hoc analysis has been conducted from the real-life ANANKE study, in which the clinical characteristics and outcomes in naïve and bio-experienced SEA patients treated with benralizumab were described for up to 96 weeks. The findings here support earlier research with a shorter treatment duration (median of 9.8 months)36 and demonstrate the sustained response of benralizumab in patients characterized by an eosinophilic-driven SA phenotype which failed to respond to previous biologics. Novel data have also been generated by analyzing bio-experienced patients split into omalizumab and mepolizumab groups. A total of four patients switched from omalizumab to mepolizumab prior to receiving benralizumab; because of the small number of patients undergoing a double biologic switch, these were combined with patients in the mepolizumab group. Within our bio-experienced group, no patients previously received dupilumab because this biologic became reimbursable in Italy after the ANANKE population was enrolled.35,38
Clinical characteristics of naïve and bio-experienced groups have already been discussed.35,37 Briefly, the eosinophilic-driven nature of SA was confirmed in all patients, with the bio-experienced group showing poorer clinical characteristics compared with naïve patients in terms of severe AER, OCS dosage, and OCS-related comorbidities. When examining the two bio-experienced subsets, we noticed that severe AER was greater in omalizumab-switched patients, while patients in the mepolizumab group showed a higher level of airway obstruction (as indicated by the lowest values of post-BD FEV1 and FVC) and higher frequency of CRSwNP. Although both benralizumab and mepolizumab induce an enhanced response in the presence of CRSwNP,19,39 our data suggest that mepolizumab may not guarantee a durable optimal control of asthma in comorbid patients, whereas benralizumab could be more efficacious. An accurate phenotyping of the comorbid patient is crucial to assist the physician in selecting the biologic treatment.40 Patients in the mepolizumab group were also characterized by a higher OCS usage compared with patients who switched from omalizumab (25 mg versus 5 mg). In line with this result, a high OCS dose is known to correlate with worse outcomes to mepolizumab treatment,39 but a superior response is expected in response to benralizumab in the eosinophilic-driven phenotype;19 interestingly, patients who take a high dose of OCS while receiving mepolizumab have recently been found to have a higher change of undergoing a biologic switch.41
Despite having different characteristics at index date, all groups considerably benefitted from benralizumab treatment, as demonstrated by the sustained improvements described across all clinical outcomes. The most impressive results are represented by the prolonged drop in any (>86%) and severe AER (≥94%), along with the substantial increase in patients with zero severe exacerbations at 96 weeks (at least 82% patients free from severe exacerbations). This dramatic decline in exacerbations coincided with a persistent BEC value of 0.0 cells/mm3 across all groups, highlighting how a broad depletion of eosinophils is necessary to achieve such a result in the context of eosinophilic-driven asthma, as already postulated by other groups.42,43 Previous long-term studies also showed a consistent exacerbations decrease in patients switching from mepolizumab to benralizumab, from zero OCS-requiring exacerbations at 48 months (N=6)31 to a 66.7% AER reduction after a median period of 31 months (AER decreased from 6 to 2);41 exacerbations were also decreased after shorter treatment periods.44,45 Similarly, patients switching from omalizumab showed a robust and significant reduction in exacerbation rate after receiving benralizumab (reduction greater than 50%28 and 90%).44
Lung function was analyzed at several time points and found to be either preserved or improved in all groups after 96 weeks of treatment. Although bio-experienced patients had a decline in both pre-BD FEV1 and FVC at 24 weeks, these parameters substantially increased at 96 weeks (+0.5 L and +0.4 L, respectively), exceeding the improvement registered in naïve patients (+0.4 L and +0.2 L, respectively). When evaluating omalizumab and mepolizumab subsets, a drop of both pre-BD parameters at 24 weeks was noticed specifically in the omalizumab group, while the mepolizumab group exhibited a striking improvement at 96 weeks. The superior respiratory response observed in mepolizumab-treated patients could be due to the poorer lung function shown by this group at index date, which may have allowed for a more pronounced response; likewise, the modest airway obstruction initially measured in omalizumab-switched patients could explain the slow and variable FEV1 increase in this subset. Nonetheless, given the remarkable decrease in AER, a recovery of respiratory parameters was expected in all groups.46,47 Once again, we can deduct that the lung function was preserved thanks to benralizumab-mediated extensive and long-lasting reduction in eosinophils, not only in peripheral blood but also in the tissues.48 As a matter of fact, benralizumab has been demonstrated to lower the number of tissue eosinophils,49 with a superior effect in decreasing sputum eosinophils when compared with mepolizumab.50 In addition, according to the novel findings reported by Bergantini et al, benralizumab induced profound changes in CD3+ T cell number and NK cell activation, and these were positively correlated with improvements in FEV1 and FVC measured in their SEA patient population.33 Therefore, this differentiating action of benralizumab is likely to explain the greater effectiveness on respiratory parameters in patients who were treated with other biologics.
In the literature, wide improvements in respiratory function were shown in patients switching to benralizumab,28,41,44 although in the 48 months-long study by Numata et al, after an initial increase in FEV1 at 24 months, a decrease of this parameter to baseline values was recorded in both naïve and mepolizumab-switched patients after 36 and 48 months of benralizumab treatment.31 Additional studies with observation periods longer than 2 years and evaluating more patients would be necessary to corroborate the findings presented here. The OCS-sparing effect of benralizumab is another essential component to evaluate its efficacy in treating SEA patients. The long-term OCS dose reduction shown in this study exceeds the results previously communicated by Caruso et al, in which naïve and bio-experienced patients reached median daily OCS doses of 4.5 mg and 8.6 mg, respectively, after a median period of 9.8 months (reductions of 61.9% and 49.1%, respectively).36 Indeed, at 48 and 96 weeks, naïve patients maintained a median OCS dose of 0 mg/daily, while bio-experienced patients had a slight increase in median OCS dose from 0 to 1.9 mg/daily. As already explained above, such a modest increment was not caused by an actual increase in OCS dosage but rather by the low number of bio-experienced patients, which further decreased at 96 weeks. In this context, it is important to emphasize that, while omalizumab-treated patients could not eliminate OCS use, patients in the mepolizumab group retained a median OCS daily dose of 0.0 mg up to 96 weeks despite receiving the highest dose at the index date (25 mg/daily). Similarly, Caminati et al also reported a durable elimination in median OCS daily dose in their mepolizumab-switched population,41 but no OCS dose reduction was found in the long-term study by Numata et al.31 Beyond the predictable conflicting results emerging from real-world studies, our findings suggest that the specific MoA of benralizumab may play a role for reaching a sustained OCS reduction of interruption. Bergantini et al recently found that higher or more frequent OCS courses may paradoxically further impair the immunological imbalance found in SEA patients by worsening NK cell dysfunction: this could be counterbalanced by benralizumab through the binding of FcγIIIRa receptors expressed on the NK cells surface, leading to a substantial restoration of NK cells maturation and activity comparable to non-asthmatic individuals.33 Previous studies focusing on omalizumab-switched patients reported either a median reduction to 0.0 mg/day,28 or a decline in OCS cycles.44 The dissimilarities in OCS reduction seen in patients switching from mepolizumab across our and previous studies are of difficult interpretation; although benralizumab has been shown to decrease OCS dosage independently of OCS duration in the PONENTE study,51 we cannot exclude that this variable could have negatively influenced OCS reduction in omalizumab-switched patients. Prolonged OCS treatment may cause OCS-related comorbidities52 and adrenal gland dysfunction; if adrenal insufficiency occurs, treatment with low-dose OCS is required independently of asthma symptoms.53 However, data on the duration of OCS treatment are not available in our and other studies, thus we can only presume that patients in the omalizumab group may have been treated with OCS for a longer period.
Both naïve and bio-experienced populations achieved asthma control already at 4 weeks after benralizumab initiation. The median ACT score rapidly increased, reaching its maximum after 96 weeks in all groups except mepolizumab-switched patients, even though this subset showed superior improvements in both respiratory function and OCS dose reduction. Unlike the mepolizumab group, omalizumab-switched patients displayed a robust improvement in asthma control, demonstrated by a median ACT score ≥20 maintained at all time points and peaking at 96 weeks. The limited change in ACT score seen in the mepolizumab group may have been caused by the modest number of patients with available ACT data. Interestingly, similar data have been shown in the 1-year study conducted by Gómez-Bastero Fernández et al, in which patients switching from omalizumab achieved a great asthma control (mean ACT score increased from 13.5 ± 5.1 to 22.9 ± 2.1), while patients switching from mepolizumab reached an inferior mean ACT score of 17.8 ± 4.7 (from an initial value of 12.4 ± 4.6).44
Based on the effectiveness of benralizumab in omalizumab-switched patients across a range of clinical outcomes, as demonstrated in our and previous studies,28,44 the eosinophilic-driven pheno-endotype of asthma appears to be often neglected in atopic patients, where the IgE-driven pathophysiological mechanism is usually considered predominant. However, asthma is a dynamic disease and T2 biomarkers have been found to fluctuate over time in SA patients even before they received a biological therapy.54 Therefore, eosinophilic inflammation may prevail and aggravate asthma symptoms in atopic SEA patients independently of IgE levels.24,42,43 That is the reason why BEC should be closely monitored in atopic patients and, ultimately, benralizumab should be the first choice for treating atopic SEA patients when they present an eosinophilic-driven asthma phenotype.
Switching asthma therapy from mepolizumab to benralizumab has become frequent in clinical practice, as indicated by the growing number of studies demonstrating a positive and durable response to benralizumab following mepolizumab failure.31,41,44,45 A possible explanation for mepolizumab failure is represented by the limited effect in reducing tissue eosinophil number compared with benralizumab:49,50 even a high dose of mepolizumab (750 mg) could decrease airway eosinophils by only 55%.55 As demonstrated in the phenotyping Mepolizumab EXacerbations in severe eosinophilic asthma (MEX) study, a large proportion of eosinophilic exacerbations occurred in SEA patients despite being on stable treatment with mepolizumab;56 on the contrary, exacerbations have been found to be mostly non-eosinophilic in benralizumab-treated patients.50 Moreover, as mentioned above, benralizumab induces a deep modulation of the immune system that goes beyond the mere apoptosis of eosinophils by leading to full recovery of NK cell maturation and activity.33 Overall, these data reinforce the hypothesis that benralizumab action within the tissue is extensive and outperforms the limited reduction of tissue eosinophils mediated by mepolizumab, thus justifying patients’ enhanced clinical response during benralizumab treatment.
Although BEC is widely used to guide the biologic choice and predict treatment efficacy, its level should be evaluated along with other clinical characteristics, which are known to be components of the eosinophilic phenotype.3 The following aspects should also be considered:
correlation between BEC and number/severity of exacerbations;
correlation between BEC and respiratory parameters;
use of OCS in-between exacerbations, and response to OCS; and
presence and clinical relevance of sensitivity to perennial allergens.
Our study’s observational retrospective design is its principal limitation. The ANANKE study has been conducted in real life; as such, the inclusion criteria (mainly related to benralizumab eligibility according to the Italian clinical practice) allowed the enrolment of a broad SEA population and determined some unavoidable heterogeneity in patients’ characteristics. In addition, since clinical characteristics at index date were gathered throughout the 12 months before benralizumab initiation, data may have been potentially skewed by the extended collection period.
Similarly to our study, some discrepancies between omalizumab and mepolizumab subsets at baseline were noticed in other real-life studies.44 Whether the initial biologic therapy determined such differences would warrant further investigations.
The lack of statistical analysis represents a considerable disadvantage of this study, as it precludes the quantitative and universal interpretation of the results. The analysis of variables was descriptive only because the primary endpoint of the ANANKE study is to describe the clinical profile of patients eligible for treatment with benralizumab in a real-world setting. As such, no formal hypotheses could be pre-specified and statistically tested.
Taking into account these limitations, the findings presented here will serve the scientific community by providing an update on the clinical outcomes observed in SEA patients switched to benralizumab after a partial response to previous biologics.
Conclusions
In this post hoc analysis, benralizumab induced a substantial and durable clinical response in both naïve and bio-experienced patients. The magnitude of the observed effects may be also due to the newly proposed MoA of benralizumab, involving improved NK cell maturation and anti-eosinophilic activity.32,33 Given the dramatic decline in AER and the broad improvement in lung function in both groups, it seems reasonable to recognize these two clinical outcomes as “treatable traits” of benralizumab in the context of eosinophilic asthma. Overall, our data not only endorse the switch to benralizumab whenever patients experience a sub-optimal response from either omalizumab and/or mepolizumab but encourage choosing benralizumab as first-line biologic to treat SEA patients whenever the eosinophilic-driven nature of asthma has been ascertained.
Acknowledgments
EDRA SpA provided writing and editorial support, with assistance from Alessandra Rossi, PhD. We thank Claudio Marchese, Sara Rizzoli, Barbara Roncari, Alessandro Zullo, and all the members of the MediNeos team for their support during the design, management, and statistical analysis of the data.
Funding Statement
AstraZeneca SpA Italy gave financial support for writing the article and participated in the study design, data collection and analysis.
Abbreviations
ACT, asthma control test; ADCC, antibody-dependent cellular cytotoxicity; AER, annual exacerbation rate; BEC, blood eosinophil count; BMI, body mass index; eCRF, electronic case report form; FeNO, fractional exhaled nitric oxide; GINA, Global Initiative for Asthma; ICS, inhaled corticosteroids; IgE, immunoglobulin E; IL5, interleukin 5; IL5Rα, interleukin 5 receptor alpha; IQR, interquartile range; LABA, long-acting β2-agonist; mAb, monoclonal antibody; MoA, mechanism of action; N, number of patients; NK, natural killer; OCS, oral corticosteroids; pre/post-BD FEV1, pre/post-bronchodilator forced expiratory volume in 1 second; pre/post-BD FVC, pre/post-bronchodilator-forced vital capacity; SA, severe asthma; SD, standard deviation; SEA, severe eosinophilic asthma.
Data Sharing Statement
Upon reasonable request, the corresponding author will provide the datasets used and/or analysed during the current work.
Ethics Approval and Informed Consent
The ANANKE study was carried out in agreement with the ethical standards outlined in the Declaration of Helsinki as well as laws and policies governing the Italian medical ethics and practice. Before participating in the study, each patient gave informed consent and signed updated informed consent and privacy forms related to the extended period of observation. Ethics committees/institutional review boards granted ethical approval at each site involved in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
PC has received grants and fees as a speaker from AstraZeneca-MedImmune, Guidotti-Malesci, and GlaxoSmithKline in the last 3 years. GWC has received research grants and lecture or advisory board fees from A. Menarini, Allergy – Therapeutics, AstraZeneca-MedImmune, Boehringer Ingelheim, Chiesi, Faes, Genentech, Guidotti-Malesci, GlaxoSmithKline, HAL Allergy, Novartis, Sanofi-Aventis, Sanofi-Genzyme/Regeneron, Stallergenes-Greer, Thermo Fisher, Valeas, and Vifor Pharma in the last 3 years. SC has received grants and/or personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Glaxo Smith Kline, Guidotti, Menarini, Novartis, and Valeas. FDMa has received lecture fees at national and international meetings and consultancy fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Dompé, Guidotti/Malesci, GlaxoSmithKline, Menarini, Novartis, and Zambon. SDG has received grants and/or personal fees from AstraZeneca, Chiesi, Glaxo Smith Kline, Menarini, Novartis, and Sanofi. GP has received lecture fees and consultancy fees from AlfaSigma, AstraZeneca, Chiesi, GlaxoSmithKline, Guidotti-Malesci, Menarini, Mundipharma, Novartis, Sanofi, and Zambon; PR has been a lecturer, speaker, and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis; her department has received funding from Almirall, Boehringer Ingelheim, Chiesi, Novartis, and Zambon. MR declares grants and personal fees from Boehringer Ingelheim, Roche, AstraZeneca, Novartis, Chiesi, GSK, Menarini, Guidotti, AlfaSigma, and Zambon. AV has received fees as a speaker/lecturer by AstraZeneca, Chiesi Farmaceutici, GSK, Novartis, and Sanofi. MB and SB are AstraZeneca employees. FM received research funding as Principal investigator by AstraZeneca, Chiesi Farmaceutici, Novartis, and Sanofi; fees as a speaker/lecturer by AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline, Novartis, and Sanofi. The authors report no other conflicts of interest in this work.
References
- 1.Bakakos A, Loukides S, Bakakos P. Severe eosinophilic asthma. J Clin Med. 2019;8(9). doi: 10.3390/jcm8091375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Groot JC, Ten Brinke A, Bel EHD. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res. 2015;1(1):00024–02015. doi: 10.1183/23120541.00024-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaney LG, Perez de Llano L, Al-Ahmad M, et al. Eosinophilic and noneosinophilic asthma: an expert consensus framework to characterize phenotypes in a global real-life severe asthma cohort. Chest. 2021;160(3):814–830. doi: 10.1016/j.chest.2021.04.013 [DOI] [PubMed] [Google Scholar]
- 4.Coumou H, Westerhof GA, de Nijs SB, Amelink M, Bel EH. Diagnosing persistent blood eosinophilia in asthma with single blood eosinophil or exhaled nitric oxide level. Respir Med. 2018;141:81–86. doi: 10.1016/j.rmed.2018.06.025 [DOI] [PubMed] [Google Scholar]
- 5.Matucci A, Maggi E, Vultaggio A. Eosinophils, the IL-5/IL-5Rα axis, and the biologic effects of benralizumab in severe asthma. Respir Med. 2019;160:105819. doi: 10.1016/j.rmed.2019.105819 [DOI] [PubMed] [Google Scholar]
- 6.Pelaia C, Paoletti G, Puggioni F, et al. Interleukin-5 in the Pathophysiology of Severe Asthma. Front Physiol. 2019;10:1514. doi: 10.3389/fphys.2019.01514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Shepard K, Yang M, et al. Overlap of allergic, eosinophilic and type 2 inflammatory subtypes in moderate-to-severe asthma. Clin Exp Allergy. 2021;51(4):546–555. doi: 10.1111/cea.13790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.2023 Gina Main Report. Available from: https://ginasthma.org/wp-content/uploads/2023/05/GINA-2023-Full-Report-2023-WMS.pdf. Accessed March 19, 2024.
- 9.European Medicines Agency. Overview of Xolair (omalizumab). EMA/375834/2020. Available from: https://www.ema.europa.eu/en/documents/overview/xolair-epar-medicine-overview_en.pdf. Accessed March 19, 2024.
- 10.European Medicines Agency. Cinqaero - Product information. Available from: https://www.ema.europa.eu/en/documents/product-information/cinqaero-epar-product-information_en.pdf. Accessed March 19, 2024.
- 11.European Medicines Agency. Nucala - Product Information. Available from: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf. Accessed March 19, 2024.
- 12.European Medicines Agency. Fasenra - Product Information. Available from: https://www.ema.europa.eu/en/documents/product-information/fasenra-epar-product-information_en.pdf. Accessed March 19, 2024.
- 13.European Medicines Agency. Dupixent - Product Information. Available from: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf. Accessed March 19, 2024.
- 14.European Medicines Agency. Tezspire - Product Information. Available from: https://www.ema.europa.eu/en/documents/product-information/tezspire-epar-product-information_en.pdf. Accessed March 19, 2024.
- 15.Kayser MZ, Drick N, Milger K, et al. Real-world multicenter experience with mepolizumab and benralizumab in the treatment of uncontrolled severe eosinophilic asthma over 12 months. JAA. 2021;14:863–871. doi: 10.2147/JAA.S319572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maglio A, Vitale C, Pelaia C, et al. Severe asthma remissions induced by biologics targeting IL5/IL5r: results from a multicenter real-life study. IJMS. 2023;24(3):2455. doi: 10.3390/ijms24032455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanario JW, Cartwright L, Jones RC, Sayers R, Hyland ME, Masoli M. “Life-changing”: the experience of super-responders to biologics in severe asthma. BMC Pulm Med. 2022;22(1):445. doi: 10.1186/s12890-022-02241-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pepper AN, Hanania NA, Humbert M, Casale TB. How to Assess Effectiveness Of Biologics For Asthma And What Steps To Take When There Is Not Benefit. J Allergy Clin Immunol. 2021;9(3):1081–1088. doi: 10.1016/j.jaip.2020.10.048 [DOI] [PubMed] [Google Scholar]
- 19.Bleecker ER, Wechsler ME, FitzGerald JM, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. 2018;52(4):1800936. doi: 10.1183/13993003.00936-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Amato M, Menzella F, Altieri E, et al. Benralizumab in patients with severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: an ANANKE Study post-hoc Analysis. Front Allergy. 2022;3:881218. doi: 10.3389/falgy.2022.881218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison TW, Chanez P, Menzella F, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, Phase 3b trial. Lancet Respir Med. 2021;9(3):260–274. doi: 10.1016/S2213-2600(20)30414-8 [DOI] [PubMed] [Google Scholar]
- 22.Nolasco S, Crimi C, Pelaia C, et al. Benralizumab effectiveness in severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: a real-world multicenter study. J Allergy Clin Immunol. 2021;9(12):4371–4380.e4. doi: 10.1016/j.jaip.2021.08.004 [DOI] [PubMed] [Google Scholar]
- 23.Pavord ID, Deniz Y, Corren J, et al. Baseline FeNO independently predicts the dupilumab response in patients with moderate-to-severe asthma. J Allergy Clin Immunol. 2022:S2213219822013034. doi: 10.1016/j.jaip.2022.11.043 [DOI] [PubMed] [Google Scholar]
- 24.Jackson DJ, Humbert M, Hirsch I, Newbold P, Garcia Gil E. Ability of Serum IgE concentration to predict exacerbation risk and benralizumab efficacy for patients with severe eosinophilic asthma. Adv Ther. 2020;37(2):718–729. doi: 10.1007/s12325-019-01191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corren J, Castro M, O’Riordan T, et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J Allergy Clin Immunol. 2020;8(2):516–526. doi: 10.1016/j.jaip.2019.08.050 [DOI] [PubMed] [Google Scholar]
- 26.Pelaia C, Crimi C, Pelaia G, et al. Real‐life evaluation of mepolizumab efficacy in patients with severe eosinophilic asthma, according to atopic trait and allergic phenotype. Clin Exp Allergy. 2020;50(7):780–788. doi: 10.1111/cea.13613 [DOI] [PubMed] [Google Scholar]
- 27.Corren J, Ambrose CS, Griffiths JM, et al. Efficacy of tezepelumab in patients with evidence of severe allergic asthma: results from the phase 3 NAVIGATOR study. Clin Exper Aller. 2022:cea.14256. doi: 10.1111/cea.14256 [DOI] [PubMed] [Google Scholar]
- 28.Pelaia C, Crimi C, Nolasco S, et al. Switch from omalizumab to benralizumab in allergic patients with severe eosinophilic asthma: a real-life experience from Southern Italy. Biomedicines. 2021;9(12):1822. doi: 10.3390/biomedicines9121822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menzies-Gow AN, McBrien C, Unni B, et al. Real world biologic use and switch patterns in severe asthma: data from the international severe asthma registry and the US CHRONICLE Study. JAA. 2022;15:63–78. doi: 10.2147/JAA.S328653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson DJ, Burhan H, Menzies-Gow A, et al. Benralizumab effectiveness in severe asthma is independent of previous biologic use. J Allergy Clin Immunol. 2022;10(6):1534–1544.e4. doi: 10.1016/j.jaip.2022.02.014 [DOI] [PubMed] [Google Scholar]
- 31.Numata T, Araya J, Miyagawa H, et al. Effectiveness of switching biologics for severe asthma patients in Japan: a single-center retrospective study. JAA. 2021;14:609–618. doi: 10.2147/JAA.S311975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dagher R, Kumar V, Copenhaver AM, et al. Novel mechanisms of action contributing to benralizumab’s potent anti-eosinophilic activity. Eur Respir J. 2022;59(3):2004306. doi: 10.1183/13993003.04306-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergantini L, d’Alessandro M, Pianigiani T, Cekorja B, Bargagli E, Cameli P. Benralizumab affects NK cell maturation and proliferation in severe asthmatic patients. Clin Immunol. 2023;253:109680. doi: 10.1016/j.clim.2023.109680 [DOI] [PubMed] [Google Scholar]
- 34.Characterisation of Italian severe uncontrolled asthmatic patieNts key features when receiving benralizumab (ANANKE). Available from: https://clinicaltrials.gov/ct2/show/NCT04272463. Accessed March 19, 2024. [DOI] [PMC free article] [PubMed]
- 35.Menzella F, Bargagli E, Aliani M, et al. Characterization of Italian severe uncontrolled Asthmatic patieNts Key features when receiving Benralizumab in a real-life setting: the observational rEtrospective ANANKE study. Respir Res. 2022;23(1):36. doi: 10.1186/s12931-022-01952-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caruso C, Cameli P, Altieri E, et al. Switching from one biologic to benralizumab in patients with severe eosinophilic asthma: an ANANKE study post hoc analysis. Front Med. 2022;9:950883. doi: 10.3389/fmed.2022.950883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vultaggio A, Aliani M, Altieri E, et al. Long-term effectiveness of benralizumab in severe eosinophilic asthma patients treated for 96-weeks: data from the ANANKE study. Respir Res. 2023;24(1):135. doi: 10.1186/s12931-023-02439-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimbursement decree for human use of Dupixent - Italian Medicines Agency. Available from: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2020-12-09&atto.codiceRedazionale=20A06599&elenco30giorni=true. Accessed March 19, 2024.
- 39.Kavanagh JE, d’Ancona G, Elstad M, et al. Real-world effectiveness and the characteristics of a “super-responder” to mepolizumab in severe eosinophilic asthma. Chest. 2020;158(2):491–500. doi: 10.1016/j.chest.2020.03.042 [DOI] [PubMed] [Google Scholar]
- 40.Seccia V, D’Amato M, Scioscia G, et al. Management of patients with severe asthma and chronic rhinosinusitis with nasal polyps: a multidisciplinary shared approach. JPM. 2022;12(7):1096. doi: 10.3390/jpm12071096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caminati M, Marcon A, Guarnieri G, et al. Benralizumab efficacy in late non-responders to mepolizumab and variables associated with occurrence of switching: a real-word perspective. JCM. 2023;12(5):1836. doi: 10.3390/jcm12051836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraft M, Brusselle G, FitzGerald JM, et al. Patient characteristics, biomarkers and exacerbation risk in severe, uncontrolled asthma. Eur Respir J. 2021;58(6):2100413. doi: 10.1183/13993003.00413-2021 [DOI] [PubMed] [Google Scholar]
- 43.Zeiger RS, Schatz M, Dalal AA, et al. Blood eosinophil count and outcomes in severe uncontrolled asthma: a prospective study. J Allergy Clin Immunol. 2017;5(1):144–153.e8. doi: 10.1016/j.jaip.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 44.Gómez-Bastero Fernández A, Medina Gallardo JF, Delgado Romero J, et al. Effectiveness of switching to benralizumab in severe refractory eosinophilic asthma. JAA. 2022;15:727–735. doi: 10.2147/JAA.S358705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kavanagh JE, Hearn AP, d’Ancona G, et al. Benralizumab after sub‐optimal response to mepolizumab in severe eosinophilic asthma. Allergy. 2021;76(6):1890–1893. doi: 10.1111/all.14693 [DOI] [PubMed] [Google Scholar]
- 46.Soremekun S, Heaney LG, Skinner D, et al. Asthma exacerbations are associated with a decline in lung function: a longitudinal population-based study. Thorax. 2022:217032. doi: 10.1136/thorax-2021-217032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortega H, Chupp G, Bardin P, et al. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J. 2014;44(1):239–241. doi: 10.1183/09031936.00220413 [DOI] [PubMed] [Google Scholar]
- 48.Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med. 2000;161(1):64–72. doi: 10.1164/ajrccm.161.1.9809100 [DOI] [PubMed] [Google Scholar]
- 49.Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132(5):1086–1096.e5. doi: 10.1016/j.jaci.2013.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poznanski SM, Mukherjee M, Zhao N, et al. Asthma exacerbations on benralizumab are largely non‐eosinophilic. Allergy. 2021;76(1):375–379. doi: 10.1111/all.14514 [DOI] [PubMed] [Google Scholar]
- 51.Menzies-Gow A, Gurnell M, Heaney LG, et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): a multicentre, open-label, single-arm study. Lancet Respir Med. 2022;10(1):47–58. doi: 10.1016/S2213-2600(21)00352-0 [DOI] [PubMed] [Google Scholar]
- 52.Chan JSK, Murray RB, Price D. Oral corticosteroids in asthma and beyond: moving forward. Eur Respir J. 2022;60(3):2200776. doi: 10.1183/13993003.00776-2022 [DOI] [PubMed] [Google Scholar]
- 53.Gurnell M, Heaney LG, Price D, Menzies‐Gow A. Long‐term corticosteroid use, adrenal insufficiency and the need for steroid‐sparing treatment in adult severe asthma. J Intern Med. 2021;290(2):240–256. doi: 10.1111/joim.13273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Zhang Q, Wang J, et al. Variability of Type 2 inflammatory markers guiding biologic therapy of severe asthma: a 5-year retrospective study from a single tertiary hospital. World Allergy Organ J. 2021;14(9):100547. doi: 10.1016/j.waojou.2021.100547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti–interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167(2):199–204. doi: 10.1164/rccm.200208-789OC [DOI] [PubMed] [Google Scholar]
- 56.McDowell PJ, Diver S, Yang F, et al. The inflammatory profile of exacerbations in patients with severe refractory eosinophilic asthma receiving mepolizumab (the MEX study): a prospective observational study. Lancet Respir Med. 2021;9(10):1174–1184. doi: 10.1016/S2213-2600(21)00004-7 [DOI] [PubMed] [Google Scholar]