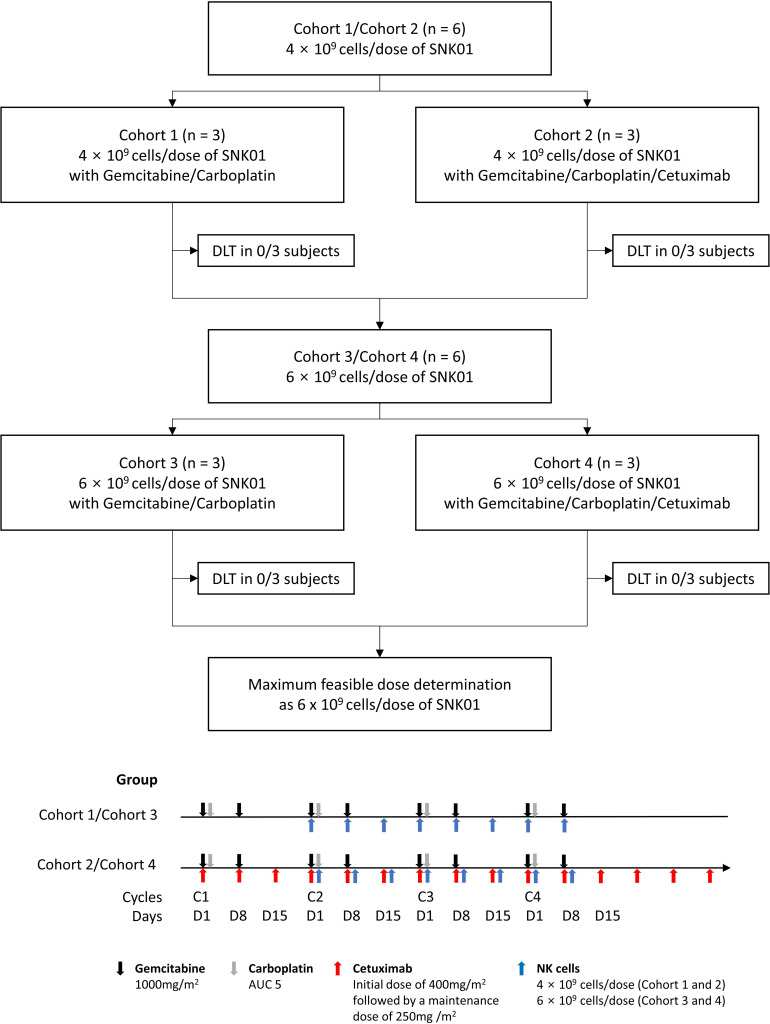
Figure 2.
Flow chart of the clinical study in patients with NSCLC receiving SNK01 (NK cells) in combination with either gemcitabine/carboplatin or gemcitabine/carboplatin/cetuximab. Three patients were allocated to each cohort, and dose escalation followed the “3+3” design. A total of 12 patients who failed prior TKI therapy were finally enrolled. During the dose-limiting toxicity (DLT) evaluation period, no DLTs were observed. As the maximum planned dose of this study was found to be tolerable, the maximum tolerated dose was not determined, and a 6×109 cells/dose was set as the maximum feasible dose. AUC, area under the concentration; NK cell, natural killer cell; NSCLC, non-small cell lung cancer; TKI, tyrosine kinase inhibitor.